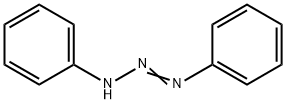
Uses of Diazoaminobenzene
Diazoaminobenzene (DAAB) is an aromatic amine that is a suspected carcinogen. It is harmful if inhaled, ingested, or absorbed through the skin. It causes skin irritation and severe irritation to eyes. DAAB can be made by diazotizing aniline dissolved in hydrochloric acid with sodium nitrite and then adding a concentrated solution of sodium acetate. DAAB is listed in the US Environmental Protection Agency’s Toxic Substances Control Act Inventory.
DAAB has three major use areas: intermediate, complexing agent, and polymer additive. Use as an intermediate is reported in several industry sectors, including organic synthesis, dye manufacture, and agrochemical manufacture (insecticides). DAAB is also a versatile metal complexing agent. A series of metabolism studies in rodents and human liver slices, electron spin resonance spectroscopy studies, short-term dermal toxicity studies in rodents, and acute bone marrow micronucleus studies in mice demonstrated that DAAB is metabolized and shares similar genotoxic and toxicological properties to the known human carcinogen, benzene, and the known rodent carcinogen, aniline.
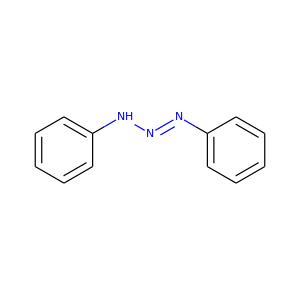
Uses
Diazoaminobenzene(DAAB)is used as a chemical intermediate, a complexing agent, and as a polymer additive. DAAB has been used to promote adhesion of natural rubber to steel tire cords. It has also been used as a blowing agent in the production of a foamed polymeric material. In addition, DAAB is used in the manufacture of dyes and insecticides. DAAB is also an impurity in certain color additives used in cosmetics, food products, and pharmaceuticals. In addition, it has been reported to show semiconducting properties and to be useful as a dopant for poly methylmethacrylate in semiconductor manufacture.
Environmental Fate
Diazoaminobenzene is an aromatic amine that exists as small golden yellow crystals at room temperature. It is insoluble in water (water solubility 0.5 g l-1) but freely soluble in ethyl alcohol, ethyl ether, benzene, pyridine, and hexane. It is stable under normal temperatures and pressures. DAAB melts at 98°C, decomposes at 130°C with major decomposition at 188°C, and explodes at its boiling point of 150°C. When heated to decomposition, it emits toxic fumes of NOx. The decomposition products of DAAB include benzene, o- and p-aminodiphenyl, diphenylamine, and azobenzene.
Mechanism of Toxicity
Diazoaminobenzene is a respiratory tract, skin, and eye irritant. DAAB yields benzene and aniline as metabolites. The proposed metabolic pathway forDAAB is that it is cleaved reductively by liver enzymes or gut flora to form aniline, benzene, and nitrogen. DAAB metabolism also results in the formation of a reactive phenyl radical, which could account for an additional risk of toxicity or carcinogenicity. The erythrocyte and lymphoid systems are major targets of DAAB toxicity. Induction of lymphoid atrophy of the thymus and other lymphoid tissues were observed, as well as methemoglobin formation, accompanying anemia, increased spleen weights, and regenerative hematopoiesis. Analysis of organ weights indicated possible chemical-related effects in the thymus, heart, spleen, kidney, and liver of rats and/or mice. Increases in the incidences of several skin lesions, including hyperplasia of the epidermis and hair follicles, and inflammation in rats and mice and ulceration in female mice were observed.
You may like
Lastest Price from Diazoaminobenzene manufacturers

US $1.00/KG2020-01-10
- CAS:
- 136-35-6
- Min. Order:
- 1KG
- Purity:
- 98% HPLC
- Supply Ability:
- 10 tons/month