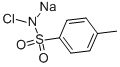
Uses of Chloramine-T

Reactions
Chloramine-T contains active (electrophilic) chlorine. Its reactivity is similar to that of sodium hypochlorite. Aqueous solutions of chloramine-T are slightly basic (pH typically 8.5). The pKa of the closely related N-chlorophenylsulfonamide C6H5SO2NClH is 9.5.
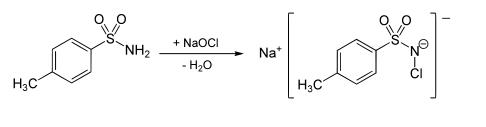
It is prepared by oxidation of toluenesulfonamide with sodium hypochlorite, with the latter being produced in situ from sodium hydroxide and chlorine (Cl2):
Uses
Chloramine-T trihydrate is used as an intermediate in the manufacture of chemical substances such as pharmaceuticals. It combines with iodogen or lactoperoxidase and is commonly used for labeling peptides and proteins with radioiodine isotopes. Hypochlorite released from chloramine-T acts as an effective oxidizing agent for iodide to form iodine monochloride (ICl).
Oxidant
Chloramine-T is a strong oxidant. It oxidizes hydrogen sulfide to sulfur and mustard gas to yield a harmless crystalline sulfimide.
Solubility
Soluble in water and ethyl alcohol. Insoluble in benzene and ethers.
Related articles And Qustion
See also
Lastest Price from Chloramine-T manufacturers
US $0.00-0.00/kg2025-11-11
- CAS:
- 127-65-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $1.00/PCS2025-04-21
- CAS:
- 127-65-1
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 10 mt