Uses and toxicity of Torcitabine
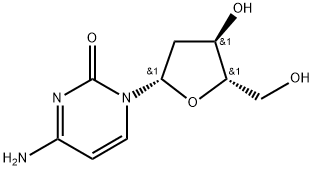
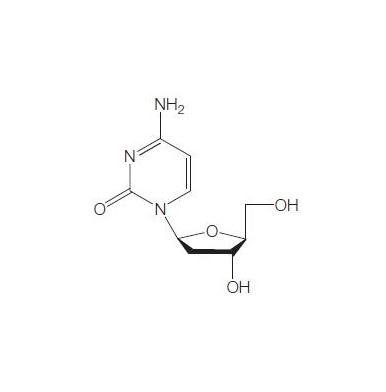
Torcitabine (b-L-2u-deoxycytidine) (L-dC) is the b-L-enantiomer of the natural nucleoside D-cytidine. The drug is currently under development by Idenix as an antiviral agent for the treatment of chronic hepatitis B virus infection. Torcitabine has poor oral bioavailability, but its 3u,5u-divaline ester (val-L-dC) and the 3u-monovaline ester, valtorcitabine dihydrochloride, have excellent oral bioavailability and consequently the torcitabine prodrug, valtorcitabine, has replaced torcitabine in clinical development.
Uses
Torcitabine and its prodrug, valtorcitabine, are investigational drugs being developed solely for the treatment of chronic HBV infection. Torcitabine itself has undergone preliminary phase I/II clinical trials in HBeAg-positive patients, initially as the 3u,5u-divalyl prodrug in escalating doses of 50, 100, 200, and 400 mg/day, but later as the more stable monovaline ester form, valtorcitabine dihydrochloride, and administered to four cohorts in doses of 300, 600, 900, and 1200 mg/ day for 28 days.
Mechanism of action
The prodrug of torcitabine, valtorcitabine, is rapidly converted to torcitabine by human esterases in plasma or the intestinal mucosa. Torcitabine, like other nucleoside and nucleotide analogs, is itself a prodrug which must be phosphorylated intracellularly to the triphosphate before it is active. Torcitabine undergoes a complex intracellular phosphorylation pathway. In both HepG2 cells and primary human hepatocytes, it is efficiently metabolized to the active form, torcitabine 5u-triphosphate (L-dCTP), by deoxycytidine kinase and a maximal intracellular concentration of 70 mM is reached in 24 hours.
In addition to L-dCTP, a second triphosphate is formed following the metabolism of L-dC-5u-monophosphate to L-dUMP by deoxycytidylate deaminase. Torcitabine triphosphate is a selective inhibitor of the polymerase enzyme of HBV. In contrast, torcitabine triphosphate concentrations r100 mM do not inhibit the enzymatic activity of human DNA polymerases a, b, or g. Like other nucleoside analogs, torcitabine most likely acts by two mechanisms, competitively inhibiting incorporation of natural nucleoside triphosphates into DNA by the HBV polymerase, and, once torcitabine triphosphate is incorporated into nascent viral DNA, by irreversibly terminating DNA synthesis (the complex of the HBV polymerase and the viral DNA with an incorporated terminal torcitabine diphosphate is very stable). The possible effects of torcitabine on virion assembly and RNaseH activity are being investigated.
Method of administration and dose
Valtorcitabine has replaced torcitabine during early clinical trials as an antiviral agent for chronic hepatitis B infection. The optimal dose has not been established, nor has the effect of co-administration with food or of its use in patients with impaired hepatic or renal function. It is being developed solely for oral administration.
Bioavailability
Torcitabine has poor bioavailability of approximately 16% in cynomologous monkeys and 10% in woodchucks. However, the absorption and bioavailability of the 3u,5u-divaline ester (val-L-dC) and the 3u-monovaline ester (valtorcitabine) are greatly improved, and both prodrugs are efficiently converted to torcitabine. In monkeys, the bioavailability of the 3u,5u-divaline ester (val-L-dC) is 70% compared with 84% for valtorcitabine. Following the intravenous administration of 3H-L-dC (10 mg/kg) as a single dose to woodchucks, the total clearance and plasma half-life (t1/2) of torcitabine were estimated to be 0.3870.33 l/h/kg and 3.170.12 hours, respectively and the t1/2 of the valine esters was similar to L-dC.
Intracellularly, L-dCTP concentrations are up to 100 times the EC50 of HBV, and the intracellular half-life of L-dCT is greater than 15 hours. Intracellular concentrations of L-dCTP remained above the 90% inhibitory concentration (IC90) (5mM) and IC50 (0.24–1.82 mM) values for WHV DNA polymerase. In primary human hepatocytes, the 5u-triphosphate concentrations reach 90.1736.4 pmol/cell.
Toxicity
Human data regarding toxicity of torcitabine and valtorcitabine are currently extremely limited. Exposure of HepG2 cells to up to 10 mM of L-dC for 14 days had no effect on the production of lactic acid, mitochondrial DNA content or mitochondrial morphology, and toxicity in bone marrow progenitor cells was not been observed. In genotoxicity studies, L-dC (in concentrations of up to 5000 mg/plate) was not mutagenic in Salmonella typhimurium and Escherichia coli plate incorporation mutagenicity assays. In mice, L-dC in doses of up to 200 mg/kg did not result in chromosomal or clastogenic abnormalities.
You may like
US $1.00-100.00/KG2024-08-19
- CAS:
- 40093-94-5
- Min. Order:
- 1KG
- Purity:
- 95%
- Supply Ability:
- 300KG