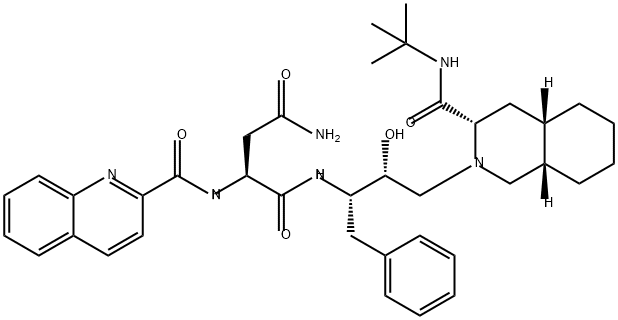
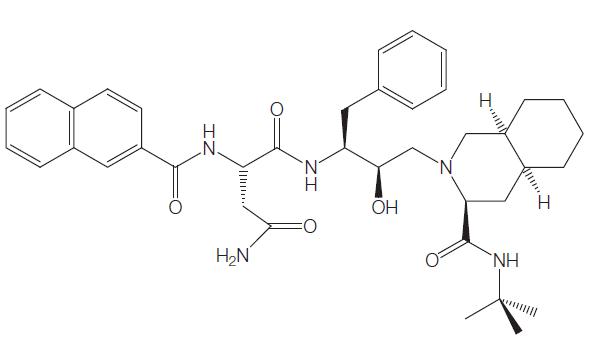
Uses and toxicity of Saquinavir
Saquinavir, a synthetic peptidomimetic analog that inhibits HIV protease, was developed using computer-led rational design technology and was the first commercially available antiretroviral protease inhibitor. It is a specific inhibitor of HIV-1, HIV-2, and simian immunodeficiency virus (SIV) proteases. Formerly known as Ro31- 8959, saquinavir, whose generic name is saquinavir mesylate, was developed by Roche and is marketed under the trade name Invirases.
Uses
Saquinavir, whether boosted or unboosted, is used only for the treatment of HIV infection.
Mechanism of action
Saquinavir was designed as a peptide-like structural mimetic of the transition state which occurs during cleavage of the Gag and Gag–Pol precursor proteins. These sites are cleaved only by the HIV protease, although a common amino acid sequence at each cleavage site is not apparent. Thus, saquinavir can sit within the active site of the HIV protease and inhibit the activity of the enzyme. Unlike the reverse transcriptase inhibitors, protease inhibitors including saquinavir do not require metabolic activation within the cell in order to exert their inhibitory effects on HIV replication.
The HIV protease belongs to the family of aspartic proteases, which includes cathepsin D and E, pepsin, renin, and gastricsin, and which can be inhibited in cell culture by the prototypic aspartic protease inhibitor pepstatin. The HIV protease is a dimer, whereas the nonviral proteases of this class are monomeric. The active site is formed at the dimer interface, with one aspartyl residue from each subunit contributing to protease activity.
The highly conserved Asp- Thr(Ser)-Gly sequence (DGT motif) within the HIV protease has been shown to be homologous to the catalytic site of proteases of the aspartic family, resulting in the inclusion of the HIV protease in this class of enzymes. Saquinavir is specific for HIV protease and therefore does not inhibit renin, pepsin, cathepsins D or E, and gastricsin at 10 mM concentration, or any of the serine or cysteine proteases. However, saquinavir and some other HIV protease inhibitor inhibitors can inhibit human proteasomes. This might explain the inhibitory effects of protease inhibitors on lymphocyte apoptosis.
Bioavailability
The doses of oral saquinavir for the first dose-ranging study were chosen to achieve a plasma concentration of 12 ng/ml, based on in vitro estimates of the IC90. For patients receiving the 600 mg three times daily dose, steady-state plasma concentrations were five times the target concentration in 11 of 12 patients, remaining above this value for the entire dosing interval in 10/12 patients. For those patients receiving 200 mg three times daily, saquinavir was detectable in plasma for the duration of the dose interval in the majority of patients, but not in those receiving 25 mg three times daily. The drug has nonlinear pharmacokinetics when given in doses of 75, 200, and 600 mg administered three times daily. There was evidence of drug accumulation (2- to 3-fold) over a period of 4 weeks.
Drug interactions
Saquinavir is both a substrate and weak inhibitor of the cytochrome P450 enzyme system. It is subject to interactions by compounds which inhibit or induce CYP3A4 as it is primarily metabolized by the CYP3A4 isoenzyme in the liver and gut. Saquinavir also inhibits the metabolism of certain 3A4 substrates, although is a significantly less potent inhibitor than ritonavir in vitro. It is also an inhibitor of the polymorphically expressed CYP3A5. An in vitro study found saquinavir to be an inhibitor of P-glycoprotein, although less potent than most other protease inhibitors.
Toxicity
Saquinavir is extremely well tolerated, with no major adverse reactions reported when used alone. Some nausea, diarrhea, and abdominal discomfort have been reported in less than 5% of study participants. Modest elevation of hepatic transaminases has also been noted in some patients. In a randomized double-blind dose-ranging study in 49 HIV-infected asymptomatic individuals who were zidovudine naive, doses of saquinavir of up to 600 mg three times daily for 16 weeks were not associated with any serious adverse events. In a phase II trial, saquinavir was not associated with any severe adverse reactions.
Lastest Price from Saquinavir manufacturers
US $1.10/g2021-07-16
- CAS:
- 127779-20-8
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min

US $15.00-10.00/KG2021-07-13
- CAS:
- 127779-20-8
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton