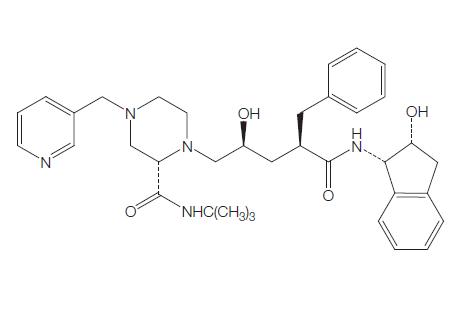
Mechanism of action of Ritonavir
Ritonavir is a peptidomimetic inhibitor of HIV-1 and HIV-2 proteases. It is marketed by Abbott Laboratories under the brand name Norvir. Ritonavir is a powerful antiretroviral agent in its own right, active against both HIV-1 and HIV-2, but its use has altered over time. Ritonavir is principally used in HIV therapy as a pharmacologic enhancing agent, that is, to increase or boost drug levels of other protease inhibitors (PIs).The concentration of the drug can be expressed as either mM or mg/ml, and 1 mM ritonavir is approximately equivalent to 0.7 mg/ml.
Uses
Ritonavir is indicated for the treatment of HIV-infected persons, usually in combination with nucleoside analogs and another protease inhibitor. Ritonavir is a powerful antiretroviral agent in its own right, but owing to some problematic adverse effects and drug interactions,its use has altered. Ritonavir is now used only as a boosting agent; that is, it is used to increase levels of other protease inhibitors.
Mechanism of action
As the HIV protease acts as a homodimer with near (or approximate) C2 symmetry, researchers at Abbott Laboratories considered that a symmetric protease inhibitor should be able to be accommodated at the active site. They developed and evaluated a series of compounds and chose ritonavir for clinical development based on its antiviral potency and favorable pharmacokinetics. Ritonavir is a C2-symmetry-based thiazole inhibitor.
Ritonavir is a competitive inhibitor of the purified aspartic acid proteases of both HIV-1 and HIV-2, with Ki values of 0.36 and 3.7 nM, respectively. At concentrations of 40 mM, there is no significant inhibition of mammalian aspartic acid proteases; thus, ritonavir is specific for the viral enzyme. Through inhibition of the HIV protease enzymes, ritonavir causes defective processing of the HIV Gag–Pol polyprotein precursor, and thus leads to the production of noninfectious (immature) HIV particles.
Ritonavir plays a major role in HIV therapy as a pharmacologic enhancing agent. Because the drug inhibits the action of many cytochrome P450 (CYP450) enzymes, it can be used in a low dose to increase (boost) blood levels of other protease inhibitors.
Excretion
The cytochrome P450 pathway is the major pathway of metabolism of ritonavir, with CYP3A4 being the predominant isoenzyme involved; CYP2D6 is involved to a lesser extent. Five metabolites have been identified, with the isopropylthiazole oxidation derivative being the principal metabolite. Although this derivative has antiviral potency similar to that of the parent compound, the plasma levels are low and AUC is approximately 3% of the parent drug, suggesting that it does not contribute significantly to the antiviral activity of ritonavir.
Human studies with radiolabeled ritonavir have demonstrated that the elimination of ritonavir is primarily via the hepatobiliary system. Fecal excretion accounts for about 86%, and approximately 11% of the drug is excreted in urine. About 34% and 3.5% of a 600-mg dose is excreted as unchanged drug in the feces and urine, respectively.
Side effects
The most frequently experienced adverse events of (full) treatmentdose ritonavir (600 mg twice daily) are nausea, vomiting, diarrhea, taste perversion, and circumoral paresthesia. The gastrointestinal side-effects of ritonavir are almost universally experienced. The most common laboratory abnormalities are elevated serum aminotransferase levels and elevated cholesterol and triglyceride levels.
Nephrotoxicity
A retrospective analysis revealed that 12 of 87 (14%) HIV-positive patients taking ritonavir developed renal insufficiency after a period of 8 days to almost a year after starting the drug. The authors comment that ten patients may have had other contributing factors (drugs, sepsis, dehydration), but recommended close monitoring of any patient taking ritonavir who may have additional risk factors for renal dysfunction. In addition, there have been a number of reports of acute renal failure, and a marked increase in serum creatinine on reintroduction was found in four of eight cases.
Related articles And Qustion
Lastest Price from Ritonavir manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 155213-67-5
- Min. Order:
- 1KG
- Purity:
- 98%-102% HPLC
- Supply Ability:
- 100kgs

US $0.00/Kg/Bag2025-04-21
- CAS:
- 155213-67-5
- Min. Order:
- 2Kg/Bag
- Purity:
- USP, GMP
- Supply Ability:
- 20tons