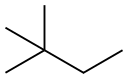
Uses and Synthesis of 2,2-Dimethylbutane
2,2-Dimethylbutane, trivially known as neohexane at William Odling's 1876 suggestion. It is therefore an alkane, indeed the most compact and branched of the hexane isomers—the only one with a quaternary carbon and a butane (C4) backbone.
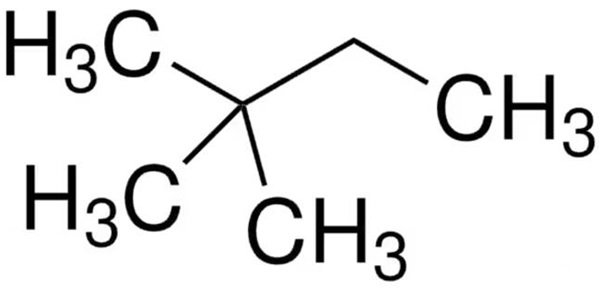
Synthesis
2,2-Dimethylbutane can be synthesised by the hydroisomerisation of 2,3-dimethylbutane using an acid catalyst.
It can also be synthesised by isomerization of n-pentane in the presence of a catalyst containing combinations of one or more of palladium, platinum, rhodium and rhenium on a matrix of zeolite, alumina, silicon dioxide or other materials. Such reactions create a mixture of final products including isopentane, n-hexane, 3-methylpentane, 2-methylpentane, 2,3-dimethylbutane and 2,2-dimethylbutane. Since the composition of the final mixture is temperature dependant the desired final component can be obtained choice of catalyst and by combinations of temperature control and distillations.
Uses
2,2-dimethylbutane (neohexane) is used as a probe molecule of metal catalysts. Probe molecules can be used to complement surface characterisation techniques for elucidating the nature of possible active sites in supported metal catalysts. 2,2-dimethylbutane (neohexane) is eminently suitable for this purpose because it combines an isobutyl and an ethyl group, representing two archetypes for hydrocarbon reactions on metal catalysts.
For 2,2-dimethylbutane, hydrogenolysis is the main reaction on most catalysts. Isomerization is restricted to platinum, palladium, and to some extent to iridium and nickel. The relative importance of isomerisation versus hydrogenolysis has been examined and it is shown that this is critically dependent on a number of factors, such as the dispersion of the metal, alloy formation, non-metallic promoters, etc. The patterns of hydrogenolysis products can also be very informative because of the relationship between the observed product and the mode of adsorption of the 2,2-dimethylbutane molecule on different types of surface site.
Lastest Price from 2,2-Dimethylbutane manufacturers
US $100.00-1.00/KG2024-03-25
- CAS:
- 75-83-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available