Uses and side effects of Nelfinavir
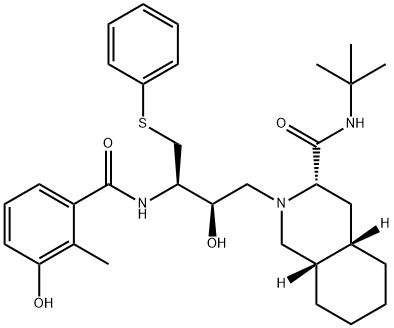
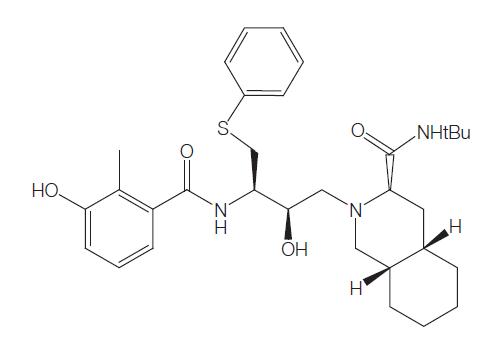
Nelfinavir mesylate is a selective, nonpeptidic inhibitor of the HIV protease, conferring moderately potent activity against HIV-1 and HIV-2. The drug was designed by protein structure-based techniques using iterative protein crystallographic analysis. The mesylate salt was chosen for development based on favorable preformulation studies. Nelfinavir inhibits the HIV protease, resulting in the formation of immature and noninfectious particles by inhibiting the cleavage of the Gag- and Gag–Pol precursor polyproteins. It was developed by Agouron Pharmaceuticals and given the trade name Viracepts.
Uses
Nelfinavir is used primarily for the treatment of HIV-1 infection, but has also been recently used in patients with malignancy. Nelfinavir is somewhat notable in that it has moderately good antiviral activity with a unique resistance profile and offers some protection regarding crossresistance to other protease inhibitors. Patients who develop resistance to nelfinavir can respond to salvage therapy with other specific drugs in this class. Several other protease inhibitors have been developed which now have advantages over nelfinavir, particularly with respect to potency. However, nelfinavir remains useful as part of combination therapy, especially in individuals who cannot tolerate boosting with ritonavir.
Mechanism of action
Nelfinavir inhibits the HIV protease with an identical mode of action to other protease inhibitors. Cleavage of Gag and Gag–Pol polyproteins is prevented through inhibition of HIV protease by nelfinavir and other drugs of this class. This results in the production of immature HIV virions.
Bioavailability
When taken with food, the bioavailability of nelfinavir is between 80% and 90%. Bioequivalence in terms of AUC and Cmax has been demonstrated between the 625-mg tablet and the 250- mg tablet of nelfinavir at a dose of 1250 mg in the fed state in 50 individuals. Nelfinavir in serum is extensively protein bound (W98%). The affinity of nelfinavir for plasma proteins alpha-1-acid glycoprotein (AAG) and serum albumin is higher than that of its metabolite M8. Both nelfinavir and M8 have higher affinity for AAG than albumin.
Side effects
The safety of nelfinavir has been assessed in a range of studies in HIV-infected individuals receiving the drug as monotherapy or in combination with nucleoside analogs. It has generally been regarded as a drug with a relatively tolerable profile and may be of benefit in individuals who require a protease inhibitor as part of their drug regimen, but who cannot tolerate ritonavir.
Adverse events occurring less frequently include gastrointestinal symptoms other than diarrhea (nausea, dyspepsia, epigastric pain, etc.), musculoskeletal symptoms, and rash. The most commonly reported laboratory abnormality is mild anemia and mild leukopenia/ neutropenia.
In individuals with HIV and hepatitis C virus coinfection who are treated with nelfinavir, the incidence of severe hepatotoxicity is low. Among protease inhibitors, nelfinavir and indinavir are considered to have the lowest rates of severe hepatotoxicity. Nelfinavir has minimal or no effect on unconjugated bilirubin levels.
A class-specific effect of bleeding in hemophiliacs, including spontaneous hemarthrosis, has been less frequently associated with nelfinavir than with other protease inhibitors.
No increase in the expected frequency of birth defects has been reported in individuals receiving nelfinavir during pregnancy.
Lastest Price from NELFINAVIR manufacturers

US $0.00-0.00/KG2025-11-28
- CAS:
- 159989-64-7
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS
US $1.10/g2021-07-16
- CAS:
- 159989-64-7
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min