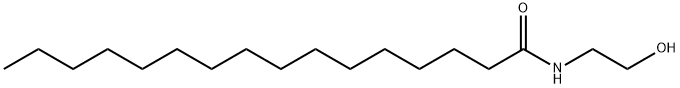
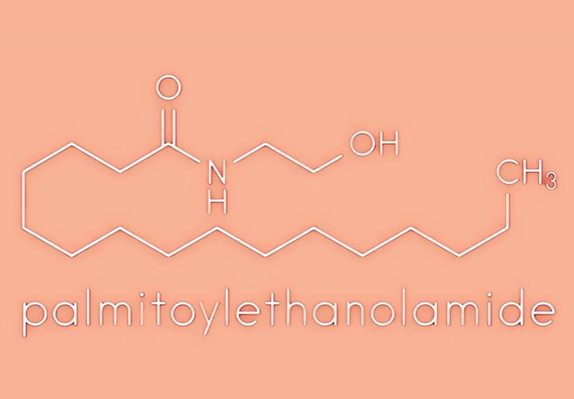
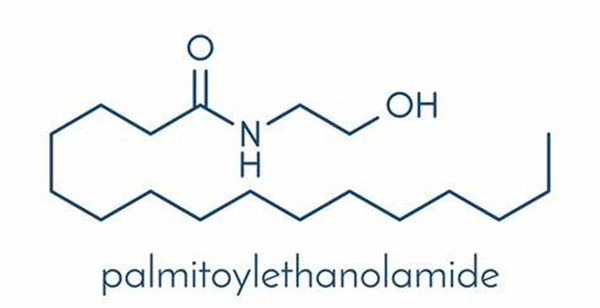
Uses and Safety of Palmitoylethanolamide
Palmitoylethanolamide (PEA) is a chemical made from fat. It is found naturally in foods such as egg yolks and peanuts, and in the human body.

Uses
PEA can bind to cells in the body and reduce pain and swelling.PEA is used for different types of pain, fibromyalgia, osteoarthritis, multiple sclerosis (MS), carpal tunnel syndrome, autism, and many other conditions, but there is no good scientific evidence to support many of these uses.
Research
Palmitoylethanolamide (PEA) is an endocannabinoid-like lipid mediator with extensively documented anti-inflammatory, analgesic, antimicrobial, immunomodulatory and neuroprotective effects. It is well tolerated and devoid of side effects in animals and humans. PEA’s actions on multiple molecular targets while modulating multiple inflammatory mediators provide therapeutic benefits in many applications, including immunity, brain health, allergy, pain modulation, joint health, sleep and recovery. PEA’s poor oral bioavailability, a major obstacle in early research, has been overcome by advanced delivery systems now licensed as food supplements. This review summarizes the functionality of PEA, supporting its use as an important dietary supplement for lifestyle management.
History
The history of PEA as a natural food ingredient with medicinal properties was first identified in 1943 as part of an epidemiological study focused on childhood rheumatic fever, which was noted to occur more frequently in those children who ate fewer eggs. Subsequently investigators noted that the occurrence of rheumatic fever was reduced in children fed egg yolk powder. Subsequently PEA was first identified in the 1950s as being an active anti-inflammatory agent in chicken egg yolk.
Safety
PEA is generally considered safe, and without adverse drug reactions (ADRs) or drug interactions. A 2016 study assessing safety claims in sixteen clinical trials, six case reports/pilot studies and a meta‐analysis of PEA as an analgesic, concluded that for treatment periods up to 49 days, clinical data argued against serious ADRs at an incidence of 1/200 or greater.
A 2016 pooled meta-analysis involving twelve studies found that no serious ADRs were registered and/or reported.No data on interactions with PEA have been reported. Based on its mechanism, PEA may be considered likely to interact with other PPAR-α agonists used to treat high triglycerides; this remains unconfirmed.
You may like
Related articles And Qustion
Lastest Price from Palmitoylethanolamide manufacturers

US $0.00/kg2025-09-02
- CAS:
- 544-31-0
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $5.00-0.50/KG2025-05-08
- CAS:
- 544-31-0
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS