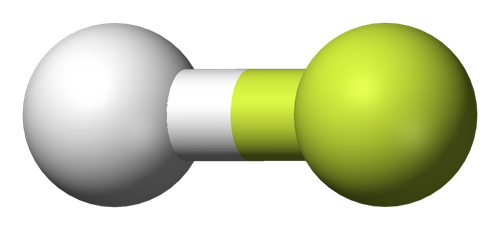Uses and production of Hydrogen fluoride
Hydrogen fluoride is a colorless, corrosive liquid or gas and is composed of a hydrogen atom and a fluorine atom. It has a strong, irritating odor. Hydrogen fluoride readily dissolves in water and is referred to as hydrofluoric acid (HFA) in its dissolved form. It is present in a variety of over-the-counter products at concentrations of 6–12%.
Structure and reactions
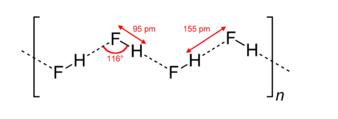
Although a diatomic molecule, HF forms relatively strong intermolecular hydrogen bonds. Solid HF consists of zig-zag chains of HF molecules. The HF molecules, with a short H–F bond of 95 pm, are linked to neighboring molecules by intermolecular H–F distances of 155 pm.Liquid HF also consists of chains of HF molecules, but the chains are shorter, consisting on average of only five or six molecules.
Comparison with other hydrogen halides
Hydrogen fluoride does not boil until 20 °C in contrast to the heavier hydrogen halides, which boil between −85 °C (−120 °F) and −35 °C (−30 °F).This hydrogen bonding between HF molecules gives rise to high viscosity in the liquid phase and lower than expected pressure in the gas phase.
Production
Hydrogen fluoride is produced by the action of sulfuric acid on pure grades of the mineral fluorite:
CaF2 + H2SO4 → 2 HF + CaSO4 The reaction is endothermic.
About 20% of manufactured HF is a byproduct of fertilizer production, which generates hexafluorosilicic acid. This acid can be degraded to release HF thermally and by hydrolysis:
H2SiF6 → 2 HF + SiF4
SiF4 + 2 H2O → 4 HF + SiO2
Uses
Hydrogen fluoride is used to make refrigerants, herbicides, pharmaceuticals, high-octane gasoline, aluminum, plastics, electrical components, and fluorescent light bulbs. Sixty percent of the hydrogen fluoride used in manufacturing is for processes to make refrigerants.
Hydrogen fluoride is also used for etching glass and metal.
Health effects
Hydrogen fluoride is one of the toxic gases produced naturally by volcanic eruptions.
Upon contact with moisture, including tissue, hydrogen fluoride immediately converts to hydrofluoric acid, which is highly corrosive and toxic. Exposure requires immediate medical attention.It can cause blindness by rapid destruction of the corneas. Breathing in hydrogen fluoride at high levels or in combination with skin contact can cause death from an irregular heartbeat or from fluid buildup in the lungs.
You may like
Related articles And Qustion
Lastest Price from Hydrogen fluoride manufacturers

US $0.00-0.00/KG2025-12-17
- CAS:
- 7664-39-3
- Min. Order:
- 1000KG
- Purity:
- 30/49/50/55/60/70%
- Supply Ability:
- 500tons

US $0.00-0.00/KG2025-12-17
- CAS:
- 7664-39-3
- Min. Order:
- 1000KG
- Purity:
- 70%
- Supply Ability:
- 500tons