Overview of hydrogen fluoride polarity
Hydrogen fluoride (fluorane) is an inorganic compound with chemical formula HF. It is a very poisonous, colorless gas or liquid that dissolves in water to yield an aqueous solution termed hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid.
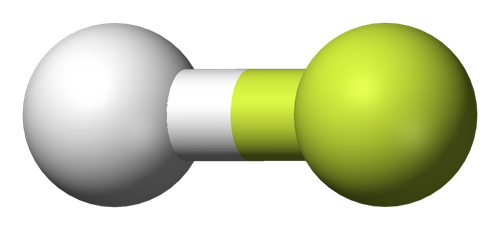
Polar molecule
Hydrogen Fluoride is a polar molecule due to the large electronegativity difference between Fluorine (3.98)and Hydrogen (2.2)which leads to induced positive charge on H atom and negative charge on F atom and therefore molecule has the net dipole.
Reactions
Like water, HF can act as a weak base, reacting with Lewis acids to give superacids. A Hammett acidity function (H0) of −21 is obtained with antimony pentafluoride (SbF5), forming fluoroantimonic acid.
Harm
Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas.
You may like
Related articles And Qustion
See also
Lastest Price from Hydrogen fluoride manufacturers

US $0.00-0.00/KG2025-09-30
- CAS:
- 7664-39-3
- Min. Order:
- 1000KG
- Purity:
- 70%
- Supply Ability:
- 500tons

US $0.00-0.00/KG2025-09-10
- CAS:
- 7664-39-3
- Min. Order:
- 1000KG
- Purity:
- 30/49/50/55/60/70%
- Supply Ability:
- 500tons