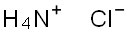USES AND APPLICATIONS OF AMMONIUM CHLORIDE
Ammonium chloride (NH4Cl), also called sal ammoniac, the salt of ammonia and hydrogen chloride. Its principal uses are as a nitrogen supply in fertilizers and as an electrolyte in dry cells, and it is also extensively employed as a constituent of galvanizing, tinning, and soldering fluxes to remove oxide coatings from metals and thereby improve the adhesion of the solders. It is a component of many proprietary cold medicines and cough remedies because of its efficacy as an expectorant, and in veterinary medicine, it is used to prevent urinary stones in goats, cattle, and sheep. Ammonium chloride is a colourless crystalline substance. It is highly soluble in water, readily forming a slightly acidic solution. It vaporizes without melting at 340 °C (644 °F) to form equal volumes of ammonia and hydrogen chloride. Ammonium chloride is yielded as a by-product in the ammonia-soda process for making sodium carbonate. It also is produced by reaction of ammonium sulfate and sodium chloride solutions. When mixed with slaked lime (calcium carbonate), ammonia gas is the result.

USES AND APPLICATIONS OF AMMONIUM CHLORIDE
Ammonium chloride can be used in various ways, its uses and most common applications are:
It is used as a diuretic for people with edema or Läennex diseases. A dose of nine grams per day is recommended. Ammonium chloride acts by increasing the renal excretion of chloride.
At the same time, also used as an acidifier, as this salt results in increased acidity concentrations of free hydrogen ions.
Ammonium chloride, handles an expectorant, helping to irritate the mucosa that is causing the stimulation of the glands of the bronchial mucosa.
In the area of pharmacokinetics, ammonium chloride, helps absorb from the GI tract for a period of five to six hours after ingestion.
Also, this salt is used for producing dry cells of tin in zinc process and galvanizing processes. In various industries it is used as flux for soldering and metal oxide remover. It is also used in textiles and pottery.
Consumption of ammonium chloride should be under medical supervision and with prior registration as it may be harmful for some people, such as those who have been diagnosed with cirrhosis or liver disease.
This salt should never be used as treatment of metabolic alkalosis because it can cause a lack of control in renal dysfunction.
If consumed without tracking established a physician can have side effects such as headache, lethargy, gastica irritation, vomiting, diarrhea, anorexia, tetany, sed, among other ailments.
Related articles And Qustion
See also
Lastest Price from Ammonium chloride manufacturers

US $1.00/g2025-04-21
- CAS:
- 12125-02-9
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 12125-02-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt