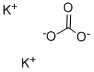
Use of Potassium carbonate
Potassium carbonate is one of the most important inorganic compounds used in industry even though it is as old as recorded history. Potassium carbonate was leached from ashes in Pompeii and mixed with slaked lime for soapmaking.

History
Potassium carbonate is the primary component of potash and the more refined pearl ash or salts of tartar. Historically, pearl ash was created by baking potash in a kiln to remove impurities. The fine, white powder remaining was the pearl ash. The first patent issued by the US Patent Office was awarded to Samuel Hopkins in 1790 for an improved method of making potash and pearl ash. In late 18th-century North America, before the development of baking powder, pearl ash was used as a leavening agent for quick breads.
Use
The potassium carbonate market is divided between the glass industry and other numerous uses. Product is shipped throughout the United States and into international regions. Video glass accounts for 44% of potassium carbonate usage, while specialty glass and ceramics use 10%. The main reason that relatively expensive potassium carbonate is used in place of soda ash in glass applications is that it is more compatible with the required lead, barium and strontium oxides.
These specialty glasses possess the improved properties of greater electrical resistivity, higher index of refraction, greater brilliance or luster, lower softening point and a wide temperature working range. In addition, potassium carbonate allows improved behavior of many colorants in glass. Potassium carbonate has a wide variety of uses outside of the glass industry.
Major uses that account for 46% of the PotCarb produced include but are not limited to: potassium silicate, pharmaceuticals, food, detergents and cleaners, photographic chemicals, agricultural, gas purification, rubber additives, polymer catalysts, potassium bicarbonate, cement and textiles.
Production
Potassium carbonate is prepared commercially by the reaction potassium hydroxide with carbon dioxide:
2 KOH + CO2 → K2CO3 + H2O
From the solution crystallizes the sesquihydrate K2CO3·3⁄2H2O ("potash hydrate"). Heating this solid above 200 °C (392 °F) gives the anhydrous salt. In an alternative method, potassium chloride is treated with carbon dioxide in the presence of an organic amine to give potassium bicarbonate, which is then calcined:
2 KHCO3 → K2CO3 + H2O + CO2
You may like
Related articles And Qustion
Lastest Price from Potassium carbonate manufacturers

US $10.00/ASSAYS2025-08-17
- CAS:
- 584-08-7
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $10.00/KG2025-04-21
- CAS:
- 584-08-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt