Manufacturing Process of Potassium carbonate
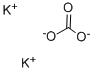
Potassium carbonate is the inorganic compound with the formula K2CO3. It is a white salt, which is soluble in water. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and glass.
Potassium carbonate was leached from ashes in Pompeii and mixed with slaked lime for soapmaking. The increase in the use of this alkali paralleled the growth of western civilization. So much wood was consumed in the production of potassium carbonate that the forests of Europe were threatened. At the time of the French Revolution, LeBlanc s invention allowed sodium carbonate to be substituted on a general basis.

Manufacturing Process
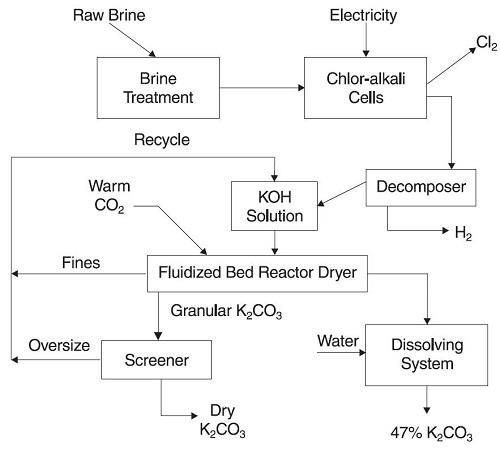
Armand Products’ Potassium Carbonate is manufactured in a fluidized bed reactor at its production facility in Muscle Shoals, AL. This results in a product that is anhydrous, making it unnecessary to perform any further processing to eliminate hydrated water (calcining). Armand Products’ Potassium Carbonate (PotCarb) is a white, dense, free-flowing granular material which is easy to handle and store.
The process starts with potassium chloride, obtained from the Canadian province of Saskatchewan. Through an electrolytic conversion of the KCl salt, potassium hydroxide (caustic potash, KOH), chlorine (Cl2) and hydrogen (H2) are produced. The hydrogen is a fuel source while the chlorine has numerous important and varied applications. Liquid caustic potash and carbon dioxide are the only raw materials required for producing PotCarb.
The dry potassium carbonate can easily be dissolved in water to form a liquid solution. Typically a 47% solution is recommended as this capitalizes on the highest concentration with the lowest freezing point (3°F). This minimizes handling problems during colder weather. The chemical equation for this process is simply:
2 KCl + 2 H2O→2 KOH + H2 + Cl2
2 KOH + CO2 →K2CO3 + H2O
The favorable logistics of this production facility cover a wide spectrum of advantages:
Largest domestic facility, utilizing four reactors in two plants;
Dry and liquid forms available in various packaging units;
Vertically integrated with onsite production of liquid caustic potash (KOH), the key raw material;
Geographical location in proximity to Gulf and Eastern coast ports, allowing for timely export shipments;
Electric power for raw material conversion and plant operations is readily available from a nearby TVA plant.
As a side note, potassium carbonate cannot be made by the Solvay process used for sodium carbonate (Na2CO3). The flow diagram for the manufacturing process is shown on the next page:
You may like
Related articles And Qustion
See also
Lastest Price from Potassium carbonate manufacturers

US $10.00/ASSAYS2025-08-17
- CAS:
- 584-08-7
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $10.00/KG2025-04-21
- CAS:
- 584-08-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt