Uranium(IV) oxide:Crystal structure,Application,Preparation
Uranium(IV) oxide is characterized by high melting point, good high-temperature stability, strong radiation resistance, good corrosion resistance, and good compatibility with zirconium or stainless steel cladding. The main disadvantage is poor thermal conductivity. It is currently the most important nuclear fuel for nuclear power reactors.
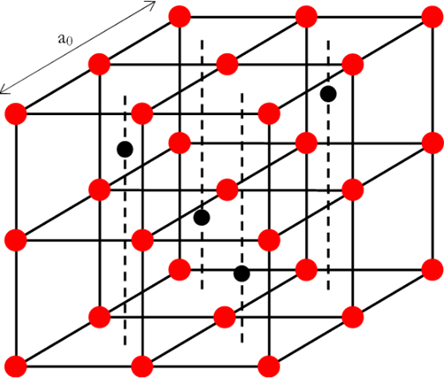
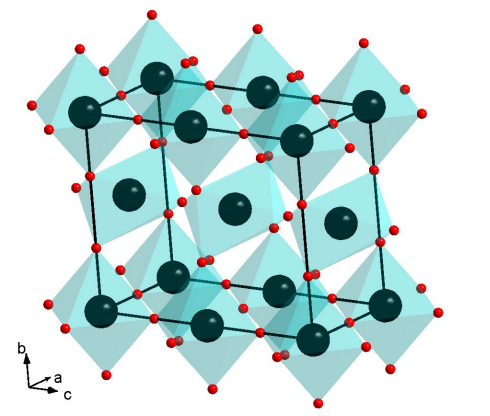
Crystal structure
Uranium(IV) oxide is an oxide of uranium. Uranium(IV) oxide crystal is composed of U4+ and O2- ions, and its crystal structure is fluorite cubic system. The O2- ions are arranged in a simple cubic sublattice, and the U4+ ions are arranged in a face-centered cubic sublattice.
Application
Uranium(IV) oxide (UO2) has the advantages of high melting point, good stability, strong radiation resistance, and low thermal neutron capture cross section. It is currently a widely used nuclear fuel in light water reactor power stations.
Preparation
Uranium(IV) oxide blocks are prepared using traditional powder metallurgy processes, which include powdering, granulating, forming, sintering, and machining.