Unveiling Doxycycline Hyclate: An In-Depth Analysis
Introduction
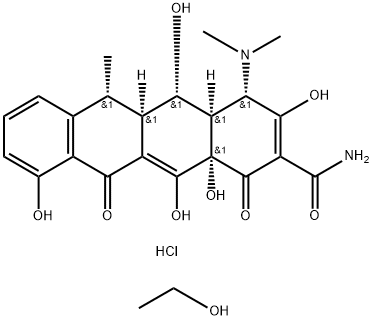
Doxycycline, a semisynthetic derivative of oxytetracycline, is a potent bacteriostatic drug commonly used as doxycycline hyclate. It possesses a broad-spectrum antimicrobial action through inhibition of protein synthesis. Additionally, it is highly lipophilic, has a large apparent volume of distribution, and has a prolonged half-life in domestic species. The plasma protein–binding rate of doxycycline hyclate is greater than the binding rate of oxytetracycline and chlortetracycline. In dogs, up to 40% of a dose of doxycycline is metabolized, and it is largely excreted in feces in the bile and intestinal secretions (< 5% and 75%, respectively), primarily in a microbiologically inactive form. Urinary excretion accounts for only 16% to 22% of each dose. Renal impairment increases intestinal excretion; thus, doxycycline does not accumulate in rats, humans, and dogs with renal failure.
Similar to the situation in other species in which doxycycline hyclate may be orally administered, parenteral administration of doxycycline hyclate may be useful to treat goats with bacterial infections, such as pneumonia, skin and soft tissue infections, urinary tract infections, salmonellosis, and colibacillosis. However, doxycycline hyclate is remarkably irritating to tissues when injected, which explains why parenteral preparations are not commonly available throughout the world.
This article will introduce a research paper from Héctor Sumano's team, they test a novel, nonirritating, long-acting formulation of doxycycline hyclate and determine its pharmacokinetics after SC injection.1

Figure 1 Characteristics of Doxycycline hyclate
Experiment
Goats were randomly allocated into 3 groups (10 goats/group). Two groups of goats received a single injection of the aqueous formulation of doxycycline hyclate (10 mg/kg); injections were administered IM in one of these groups and IV in the other group. Immediately before injection, the aqueous formulation of doxycycline (10% solution) was prepared from powdered doxycycline hyclate that was diluted in sterile distilled water. Each goat was weighed, and the dose of doxycycline (10 mg/kg in a volume of approx 5.0 mL) was administered, respectively, IV in a jugular vein or IM in a semitendinosus or semimembranosus muscle of either hind limb.
Goats in the third group received a single SC injection of an experimental long-acting formulation of doxycycline hyclate at the same dosage (ie, 10 mg/kg). A long-acting formulation of doxycycline hyclate (100 mg/mL) was prepared by use of sterile conditions. Doxycycline hyclate a was mixed with β-cyclodextrinb by use of the kneading method to form a 10% (wt:vol) doxycycline solution. First, β-cyclodextrin (0.1M) and distilled water were mixed by use of a mortar-and-pestle to obtain a homogeneous paste. Then, doxycycline hyclate (1M) was added slowly. The compound was mixed for 30 minutes, and an appropriate quantity of water was added to maintain suitable consistency of the paste. The paste was dried in an oven at 40° to 50°C for 24 hours, and the dried complex was then pulverized into a fine powder. The resulting powder was diluted with a solution of 15% propylene glycol–10% ethyl alcohol in water. This mixture was then included in a gel copolymer polyoxypropile–polyoxyethylene (ie, poloxamer),c which was adjusted to pH 7.0 by addition of PBS solution with constant stirring at 4°C to achieve a final concentration of 10% doxycycline–15% poloxamer.
Results
Goats did not have signs of pain or discomfort after injection of the long-acting formulation. In contrast, swelling and signs of pain were evident for at least 15 days after IM injection of the aqueous formulation. No inflammatory response was evident at the injection site after administration of the long-acting formulation of doxycycline hyclate.
However, a clearly defined bulge was detectable in 3 goats. These bulges had an initial size of 2 X 1 X 1 cm and gradually disappeared by day 7 after injection. The bulges did not appear to cause pain or discomfort to any of the affected goats. The bulges were not believed to be an inflammatory response; instead, they were considered to be space occupied by the poloxamer when it transformed to a gel at body temperature.
References:
[1] DINORAH VARGAS. Pharmacokinetics after administration of an injectable experimental long-acting parenteral formulation of doxycycline hyclate in goats.[J]. American journal of veterinary research, 2008, 69 8. DOI:10.2460/ajvr.69.8.1085.See also
Lastest Price from Doxycycline hyclate manufacturers
US $0.00-0.00/KG2025-12-01
- CAS:
- 24390-14-5
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00/KG2025-08-30
- CAS:
- 24390-14-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 2000KG