Tris(dimethylaminomethyl)phenol: An Overview of its Properties, Composition, Applications, and Storage
Introduction
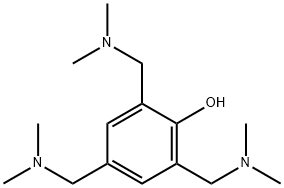
Tris(dimethylaminomethyl)phenol is a versatile chemical compound frequently used in various industrial applications, particularly in the fields of adhesives, coatings, and polymer science. Its chemical structure consists of three dimethylaminomethyl groups attached to a phenol ring, giving it unique catalytic and curing properties.

Figure 1 Characteristics of Tris(dimethylaminomethyl)phenol
Chemical Properties
Tris(dimethylaminomethyl)phenol is a clear, pale yellow to amber liquid with a characteristic amine odor. Its molecular formula is C15H27N3O, and it has a molecular weight of approximately 265.4 g/mol. The compound has a relatively low viscosity, which makes it easy to handle in liquid form. It is soluble in a wide range of organic solvents, such as alcohols, ketones, and aromatic hydrocarbons, and it demonstrates excellent stability under normal conditions.
One of the key properties of TRIS(DIMETHYLAMINOMETHYL)PHENOL is its role as a strong base and nucleophile due to the presence of the dimethylamino groups. These groups, attached to the phenolic structure, enable the compound to function as a highly efficient catalyst in many chemical reactions, particularly those involving epoxy resins and polyurethanes. Additionally, the phenol group provides further reactivity, allowing it to engage in hydrogen bonding and interact with other chemical species.
TRIS(DIMETHYLAMINOMETHYL)PHENOL also exhibits a relatively high boiling point, typically ranging between 280°C and 290°C. This high boiling point, combined with its low vapor pressure, ensures that it remains stable under high-temperature conditions commonly encountered during industrial processes. Its flashpoint is generally over 100°C, reducing the risk of ignition in regular handling environments. However, caution should be taken when working with the compound due to its moderate toxicity and potential to cause skin and eye irritation.
Composition and Structure
The chemical structure of Tris(dimethylaminomethyl)phenol is composed of a phenol ring substituted at three positions with dimethylaminomethyl groups (-CH2N(CH3)2). This structural arrangement contributes to the compound's high basicity and nucleophilicity, which in turn enhances its effectiveness as a catalyst in polymerization and crosslinking reactions. The phenol ring itself, a key component of the structure, plays a crucial role in stabilizing the compound and enabling further interactions with other chemical components during reactions.
The dimethylamino groups on the molecule act as electron-donating groups, significantly enhancing the reactivity of the phenolic hydroxyl group (-OH) in various chemical contexts. This makes the compound particularly suitable for catalytic processes that require precise control over the reaction environment. The basicity of the amine groups also allows TRIS(DIMETHYLAMINO-METHYL)PHENOL to neutralize acidic species in formulations, contributing to its versatility in industrial applications.
Main Components and Purity
TRIS(DIMETHYLAMINOMETHYL)PHENOL is typically manufactured with high purity levels to ensure its effectiveness in sensitive chemical processes. The primary component is the Tris(dimethylaminomethyl)phenol molecule itself, but depending on the production method, trace impurities may include residual solvents, unreacted amines, or minor by-products from the synthesis process. These impurities are usually controlled through purification steps to ensure the final product meets stringent industrial standards.
For most commercial applications, TRIS(DIMETHYLAMINOMETHYL)PHENOL is available in formulations with purity levels exceeding 95%. Ensuring a high degree of purity is particularly important in applications where the compound serves as a catalyst, as impurities could interfere with the efficiency of the chemical reactions. Manufacturers often provide detailed specifications regarding the purity and composition of their TRIS(DIMETHYLAMINO-METHYL)PHENOL products, which are crucial for chemists and engineers working in industries such as coatings, adhesives, and polymer manufacturing.
Applications
The primary application of Tris(dimethylaminomethyl)phenol lies in its role as a curing agent and catalyst in epoxy and polyurethane systems. In epoxy resins, TRIS(DIMETHYLAMINOMETHYL)PHENOL is used to accelerate the curing process, allowing for faster hardening of the material at ambient or slightly elevated temperatures. This property is particularly valuable in industries such as electronics, aerospace, and automotive manufacturing, where rapid curing is necessary to meet production demands.
In polyurethane systems, TRIS(DIMETHYLAMINOMETHYL)PHENOL acts as a catalyst in the formation of urethane linkages. Its basicity promotes the reaction between isocyanates and polyols, leading to the formation of polyurethane foams, elastomers, and coatings. The use of TRIS(DIMETHYLAMINOMETHYL)PHENOL in these systems allows for greater control over reaction kinetics, improving the mechanical properties and durability of the final product.
Another notable application of TRIS(DIMETHYLAMINOMETHYL)PHENOL is in the production of adhesives and sealants. The compound is frequently incorporated into adhesive formulations to enhance bonding strength and reduce cure times. Its effectiveness in these applications stems from its ability to promote crosslinking reactions between polymer chains, thereby improving the overall integrity and resilience of the adhesive material.
In addition to these primary uses, Tris(dimethylaminomethyl)phenol has found applications in other areas such as coatings, where it acts as a curing accelerator for various types of paints and varnishes. Its ability to improve drying times and enhance the chemical resistance of coatings makes it a popular choice in industrial and protective coating systems.
Storage and Handling
Proper storage and handling of Tris(dimethylaminomethyl)phenol are critical to ensuring its stability and safety. Due to its reactive nature, the compound should be stored in tightly sealed containers to prevent exposure to moisture and air. Moisture can lead to degradation of the product, potentially reducing its effectiveness as a catalyst. Furthermore, exposure to air can result in oxidation of the amine groups, which could compromise the quality of the compound.
TRIS(DIMETHYLAMINOMETHYL)PHENOL should be stored in a cool, dry place away from direct sunlight and heat sources. The recommended storage temperature is typically between 15°C and 30°C, although this can vary depending on the specific formulation. It is essential to avoid prolonged exposure to elevated temperatures, as this could lead to unwanted side reactions or degradation of the material.
When handling TRIS(DIMETHYLAMINOMETHYL)PHENOL, appropriate personal protective equipment (PPE) such as gloves, safety goggles, and lab coats should be worn to prevent skin and eye contact. The compound can irritate upon contact, and inhalation of its vapors should be avoided. In the event of accidental exposure, affected areas should be thoroughly rinsed with water, and medical attention should be sought if necessary.
References:
[1] M.D. L K, PH.D. T E M D, PH.D. R J D tech. OCCUPATIONAL ALLERGIC CONTACT DERMATITIS CAUSED BY 2,4,6-TRIS-(DIMETHYLAMINOMETHYL)PHENOL, AND REVIEW OF SENSITIZING EPOXY RESIN HARDENERS[J]. International Journal of Dermatology, 1996, 35 12: 837-896. DOI:10.1111/j.1365-4362.1996.tb05050.x.[2] M.D. L K, PH.D. T E M D, PH.D. R J D tech. OCCUPATIONAL ALLERGIC CONTACT DERMATITIS CAUSED BY 2,4,6-TRIS-(DIMETHYLAMINOMETHYL)PHENOL, AND REVIEW OF SENSITIZING EPOXY RESIN HARDENERS[J]. International Journal of Dermatology, 1996, 35 12: 837-896. DOI:10.1111/j.1365-4362.1996.tb05050.x.
You may like
Lastest Price from Tris(dimethylaminomethyl)phenol manufacturers

US $0.00/kg2025-08-26
- CAS:
- 90-72-2
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $1.00/PCS2025-04-21
- CAS:
- 90-72-2
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 10 mt