Tranexamic Acid: Pharmacological Effects and Adverse Effects
Tranexamic acid is a synthetic derivative of lysine and an antifibrinolytic drug with hemostatic effect. Tranexamic acid can strongly adsorb to the lysine binding site (LBS) of the fibrin affinity site on plasmin and plasminogen, inhibiting the binding of plasmin, plasminogen and fibrin, thereby strongly inhibiting the fibrinolytic effect caused by plasmin; in addition, when antiplasmins such as macroglobulin are present in the serum, the antifibrinolytic effect of tranexamic acid is more obvious.

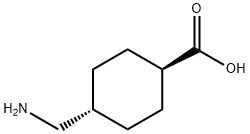
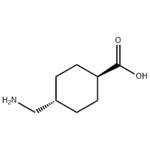
Figure 1. Tranexamic acid
Pharmacological action
Tranexamic acid can competitively inhibit the binding of plasminogen and fibrin, thereby preventing its activation. Protecting fibrin from being degraded and dissolved by plasmin, ultimately achieving a hemostatic effect. It can also inhibit the activity of plasmin and reduce the role of plasmin in activating complement, thereby preventing the occurrence of hereditary angioedema. At high concentrations, this product also has a direct inhibitory effect on plasmin.
Uses
Tranexamic acid is a protease inhibitor.
After the skin is exposed to ultraviolet light, tranexamic acid can not only inhibit tyrosinase, but also inhibit the release of prostaglandin PGE2, preventing the aggregation of melanin. Protease-activated receptor 2 (PAR-2) in keratinocytes can promote the diffusion of melanin to surrounding cells, leading to skin pigmentation and darkening. Tranexamic acid can inhibit the expression of protease-activated receptors, thereby preventing the transportation and diffusion of melanin, and exerting a whitening effect from the back end.
Adverse reactions
The most common adverse reactions of oral tranexamic acid include gastrointestinal discomfort, decreased menstruation, headache, myalgia, and palpitations, which are usually mild and short-lived. Other adverse reactions include dizziness, joint pain, blurred vision, fatigue, and hair loss.
Topical use of tranexamic acid may cause local erythema, skin irritation, dryness, and desquamation.
Intradermal injection of tranexamic acid may cause pain and hypopigmentation at the injection site, transient erythema, and skin edema.
Tranexamic acid has a potential risk of causing deep vein thrombosis, and risk factors for thromboembolic diseases should be carefully screened before use.
Related articles And Qustion
See also
Lastest Price from Tranexamic Acid manufacturers
US $0.00/kg2025-11-05
- CAS:
- 1197-18-8
- Min. Order:
- 25kg
- Purity:
- 0.99
- Supply Ability:
- /
US $0.00-0.00/kg2025-08-21
- CAS:
- 1197-18-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1