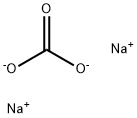
Toxicity of Sodium carbonate

Pharmacodynamics
Alkalizing buffering action: Sodium bicarbonate is an alkalinizing agent that dissociates to provide bicarbonate ion. Bicarbonate in excess of that needed to buffer hydrogen ions causes systemic alkalinization and, when excreted, urine alkalinization as well. Oral antacid action: Taken orally, sodium bicarbonate neutralizes stomach acid by the above mechanism.
Mechanism of action
Carbon dioxide from the tissues diffuses rapidly into red blood cells, where it is hydrated with water to form carbonic acid. This reaction is accelerated by carbonic anhydrase, an enzyme present in high concentrations in red blood cells. The carbonic acid formed dissociates into bicarbonate and hydrogen ions. Most of the bicarbonate ions diffuse into the plasma. Since the ratio of H2CO3 to dissolved CO2 is constant at equilibrium, pH may be expressed in terms of bicarbonate ion concentration and partial pressure of CO2 by means of the Henderson-Hasselbach equation: pH = pk + log [HCO3-]/aPCO2.
Toxicity
Man: LD50 (Oral) - 714 mg/kg, Effect: Behavioural,General Anesthetic : GI Ulceration or Bleeding from small intestine. Mouse : LC50 ( Inhalation ) - 1200mg/m3/2h : GI Other Change Mouse : LC50 ( Intraperitoneal ) - 117mg/kg Mouse : LD50 ( Oral) - 6600mg/kg Mouse : LD50 (Subcutaneous ) - 2210 mg/kg Rat : LC50 ( Inhalation ) 2300mg/m3/2H Rat: LD50 (Oral) - 4090 mg/kg.
You may like
Related articles And Qustion
Lastest Price from Sodium carbonate manufacturers

US $1200.00-1100.00/ton2025-11-07
- CAS:
- 497-19-8
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $1.00/g2025-08-19
- CAS:
- 497-19-8
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg