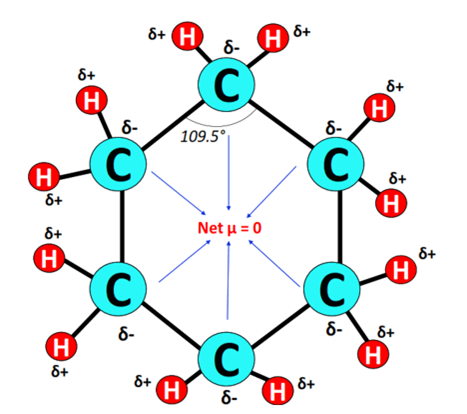
Toxicity of Cyclohexane
Cyclohexane is obtained by the distillation of petroleum or by hydrogenation of benzene. It constitutes 0.5–1.0% of petroleum.

Uses
Cyclohexane is used as a nonpolar solvent for lacquers, resins, fats, oils, and waxes, in paint and varnish remover, in the manufacture of nylon, in the extraction of essential oils, and in analytical chemistry for molecular weight determination. In addition, it is used in the manufacture of adipic acid, benzene, cyclohexanone, cyclohexanol, cyclohexyl chloride, nitrocyclohexane, and solid fuel for camp stoves. Further, it is used in industrial recrystallization of steroids and in fungicidal formulations (it has a slight fungicidal action).
Environmental Fate
If released to air, cyclohexane will exist solely as a vapor in the ambient atmosphere, and will be degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals, though direct photolysis is not expected due to the lack of absorption in the environmental spectrum. Volatilization from water surfaces is expected to be an important fate for this compound, and half-lives in a model river and lake are expected to be 3 h and ~3.5 days respectively. Adsorption to suspended solids and sediments is also expected, though hydrolysis in the environment is unlikely due to the lack of hydrolyzable functional groups.
The potential for bioconcentration and bioaccumulation of cyclohexane in aquatic organisms is moderate. It is highly resistant to biodegradation and is catabolized chiefly by cooxidation.
Mechanism of toxicity
The precise mechanism of toxicity of cyclohexane has not been
identified, but is likely similar to other central nervous system
(CNS) depressants and general anesthetics. These compounds
are believed to exert their effects through a general interaction
with the CNS, and interference with neuronal membrane
functions has been postulated as a mechanism of action.
Disruption of membrane enzymes and the corresponding
alterations in cell functions may account for the behavioral and
anesthetic effects observed following exposure to various
solvents.
You may like
Related articles And Qustion
Lastest Price from Cyclohexane manufacturers

US $10.00/KG2025-04-21
- CAS:
- 110-82-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 110-82-7
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons