How to Determine the Polarity of Cyclohexane?
Cyclohexane is a petroleum product obtainedby distilling C4- 400°F boiling rangenaphthas, followed by fractionation and superfractionation; also formed by catalytic hydrogenation of benzene. It is used extensively as a solvent for lacquers and resins, as a paint and varnish remover, and in the manufacture of adipic acid, benzene,cyclohexanol, and cyclohexanone.
Generally, solutes are soluble in solvents of similar polarity to them, and in order to use the cyclohexane solvent effectively, we need to understand its polarity, which can be judged from two aspects: bond polarity and molecular geometry.
Bond Polarity
The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (ΔEN) between the two atoms. If the electronegativity difference (ΔEN) is less than 0.4, then the bond is nonpolar covalent bond; if ΔEN is between 0.4 to 1.7,the bond is polar covalent bond; if ΔEN is greater than 1.7, the bond is an ionic bond.
For C-H bond:
The electronegativity difference (ΔEN) = 2.55(C) – 2.2(H) = 0.35
This value is less than 0.4, hence, each C-H bond is a nonpolar covalent bond, there will not be any partial positive charge (ẟ+) or partial negative charge (ẟ-) on the carbon and hydrogen atoms.
Molecular Geometry
The structure of cyclohexane is symmetrical, The carbon chain is surrounded by hydrogen atoms which are equidistant as well as at equal angles.
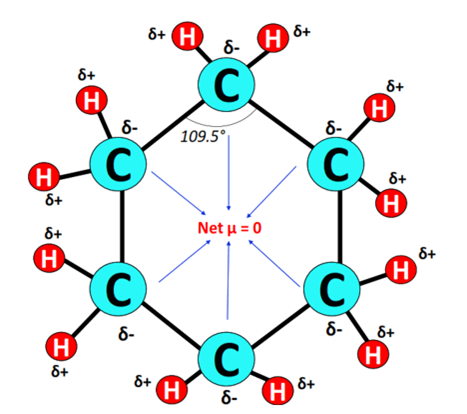
As all the 12 bonds (C-H) are nonpolar and the Cyclohexane molecule has a symmetrical geometry, there are no positive and negative poles of charges on the overall molecule of cyclohexane.
Hence, the cyclohexane (C6H12) molecule is nonpolar.
You may like
Related articles And Qustion
See also
Lastest Price from Cyclohexane manufacturers

US $10.00/KG2025-04-21
- CAS:
- 110-82-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 110-82-7
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons