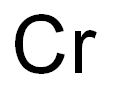Toxicity of Chromium
Chromium as a metallic element was first discovered over 200 years ago, in 1797. But the history of chromium really began several decades before this. In 1761, in the Beresof Mines of the Ural Mountains, Johann Gottlob Lehmann obtained samples of an orange-red mineral, which he called ‘Siberian red lead.’ He analyzed this mineral in 1766 and discovered that it contained lead “mineralized with a selenitic spar and iron particles.” The mineral he found was crocoite, a lead chromate (PbCrO4).

Uses
Chromium is a transitional element with many industrial uses. It is mainly used in imparting a shiny appearance to metal surfaces. In the early 1800s, the mineral, now known as chromite, was widely used in the production of paint as well as in the production of chromium compounds. These compounds can be used in a variety of applications. For example, potassium dichromate is used in the dyeing industry and chromium salts are used in leather tanning and wood preservation. Today, perhaps its most important use is in the production, in combination with iron, of stainless steel, as well as applying shiny finishes.
Environmental Fate
Chromium enters the air, water, and soil mostly in the chromium( III) and chromium(VI) forms. In air, chromium compounds are present mostly as fine dust particles, which eventually settle over land and water. Chromium can strongly attach to sediment and soil, and only a small amount is expected to dissolve in water and leach though the soil to groundwater. Fish do not accumulate much chromium in their bodies.
Most chromium exposure in the general population is through ingestion of the chemical in food containing chromium( II), although exposure is also possible as a result of drinking contaminated well water, or living near uncontrolled hazardous waste sites containing chromium or industries that use chromium. Inhalation of chromium dust and skin contact during use in the workplace are the main routes of occupational exposure.
Toxicity
Chromium may cause adverse health effects following inhalation,
ingestion, or dermal exposure. The toxicity of chromium
is mainly caused by hexavalent compounds as a result of a higher cellular uptake of chromium(VI) compounds than
chromium(III). This is explained by the fact that the chromate
anion (CrO4)2-1can enter the cells via facilitated diffusion
through nonspecific anion channels (similar to phosphate and
sulfate anions). Absorption of chromium(III) compounds is
via passive diffusion and phagocytosis.
Hexavalent chromium is unstable in the body and is reduced intracellularly (by many substances, including ascorbate and glutathione), providing very reactive pentavalent chromium and trivalent chromium. Both of these intermediates can alter DNA.
You may like
Related articles And Qustion
Lastest Price from Chromium manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7440-47-3
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $12.50-10.50/kg2025-01-16
- CAS:
- 7440-47-3
- Min. Order:
- 1000kg
- Purity:
- 99%
- Supply Ability:
- 1000ton/Year