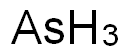Toxicity of Arsenic
The word arsenic has several derivations including from the Syriac word (al) zarniqa, the Persian word zarnikh,and the Greek word arsenikon (Arsεnikón). It is also related to the similar Greek word arsenikos, meaning ‘masculine’ or ‘potent.’ The word was subsequently adopted into Latin as arsenicum and ultimately into French and English as arsenic.

History
Arsenic compounds have been known since the days of Ancient Greece and Rome when arsenic sulfide or orpiment (As2S3) was used by physicians to heal and by murderers to poison. Arsenic compounds were mined by the early Chinese, Greek, and Egyptian civilizations who found it could be produced from its ores easily that made it one of the first recognized as an element by early alchemists. Alchemy existed from about 500 BC to about the end of the sixteenth century. Alchemists were on a quest to turn various metals into precious metals such as gold and searching for ways to have eternal life. While most of alchemy was shrouded in mysticism and magic, a number of early techniques were later found to be useful in modern chemistry. The discovery of arsenic is credited to alchemist Albert the Great (Albertus Magnus 1193–1280), a German Dominican friar who achieved fame for his advocacy in the peaceful coexistence of science and religion. He heated As2S3 with soap and formed elemental arsenic. The first directions for the preparation of the metalloid arsenic, however, are found in the writings of Paracelsus (1493–1541), the father of modern toxicology.
Uses
Dioscorides, a Greek physician, described arsenic as a poison in the first century. Its ideal properties for sinister uses included its lack of color, odor, or taste when mixed in food or drink and its easy access that made it readily available to all classes of society. The primary current use of arsenic is for strengthening alloys of copper and lead for car batteries. Arsenic is found in semiconductor electronic devices and in the production of chemicals such as chromated copper arsenate, monosodium methyl arsenate (MSMA), and disodium methyl arsenate (DSMA) used for treating wood products, herbicides, and insecticides. 4-Hydroxy-3-nitrobenzenearsonic acid is added to animal food as a method of disease prevention and growth stimulation. Arsenic compounds have been used as medicines, including arsphenamine and neosalvasan that were indicated for syphilis and typanosomiasis but have been superseded by modern antibiotics. Arsenic trioxide has been used in a variety of ways over the past 500 years, but recently in the treatment of acute promyelocytic leukemia. Potassium arsenite was used in Fowler’s solution for the treatment of psoriasis, malaria, chorea, and syphilis until the 1950s. Lewisite is an organoarsenic compound once manufactured in the United States and Japan as a chemical weapon that caused severe blistering and lung irritation.
Environmental Fate
Bioconcentration of arsenic occurs in algae and lower invertebrates. Both bottom-feeding and predatory fish can accumulate contaminants found in water. The major bioaccumulation transfer is between water and algae, at the base of the food chain that has a strong impact on the concentration in fish. Bottom-feeders are readily exposed to the greater quantities of arsenic, which accumulate in sediments. No differences were found for arsenic existing between bottom-feeders and predators in tissue levels of metals and other contaminants. Therefore, biomagnification in aquatic food chains does not appear to be significant.
Mechanism of Toxicity
Trivalent arsenic exerts its toxic effects mainly by disrupting ATP production by inhibiting lipoic acid, which is a cofactor for pyruvate as well by replacing phosphate which uncouples oxidation phosphorylation. This inhibits the electron transport chain in the mitochondria and the ultimate synthesis of ATP. Hydrogen peroxide production is also increased, which, it is speculated, has potential to form reactive oxygen species and oxidative stress. These metabolic interferences lead to death from multisystem cell death and organ failure. The activity of enzymes is due to the functional groups on amino acids such as the sulfhydryl group on cysteine or coenzymes such as lipoic acid, which has vicinal thiol groups. Trivalent inorganic arsenicals readily react with sulfhydryl groups such as cysteine creating a strong complex between arsenic and vicinal sulfhydryl reagents.
These actions inhibit not only the formation of Acetyl-CoA but also the enzymes succinic dehydrogenase and pyruvate. Arsenite inhibits the binding of steroids to the glucocorticoid receptor, but not other steroid receptors. The probable mechanism of toxicity of pentavalent inorganic arsenate is its reduction to a trivalent form, arsenite, which is more toxic than the arsenate. Thus, a variety of mechanisms lead arsenic to impair cell respiration and subsequently diminish ATP formation.
You may like
Related articles And Qustion
Lastest Price from Arsenic manufacturers
US $50.00-1.00/KG2024-03-25
- CAS:
- 7440-38-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available