Toxicity of Acetaldehyde
Description
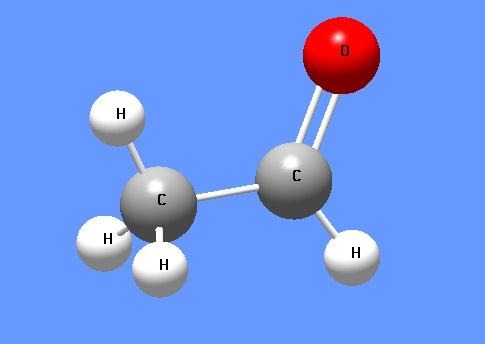
Acetaldehyde (systematic name ethanal) is an organic chemical compound with the formula CH3CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants.
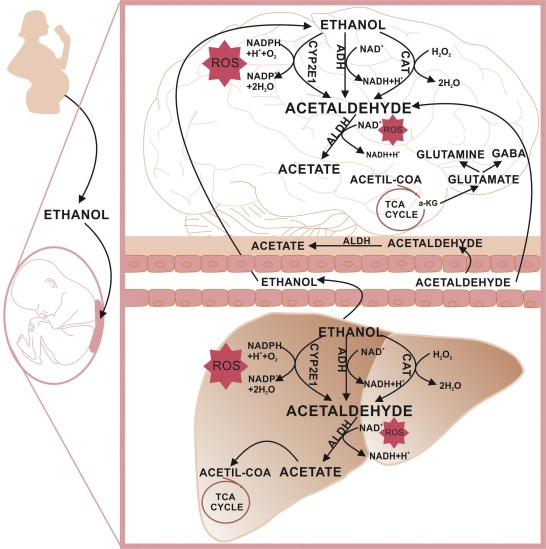
It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body.
The International Agency for Research on Cancer (IARC) has listed acetaldehyde as a Group 1 carcinogen. Acetaldehyde is "one of the most frequently found air toxins with cancer risk greater than one in a million".
Identification and Use
Acetaldehyde is a colorless volatile liquid with a pungent suffocating odor. It is not registered for current pesticide use in the U.S., but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved use. Acetaldehyde used as an intermediate in the production of cellulose acetate, vinyl acetate resins, acetate esters, synthetic pyridine derivatives, and terephthalic acid.
Other uses include: in the silvering of mirrors; in leather tanning, as a denaturant for alcohol; in fuel mixtures; as a hardener for gelatin fibers; in glue and casein products; as a preservative for fish and fruit; in the paper industry; as a synthetic flavoring agent; and in the manufacture of cosmetics. Acetaldehyde has been identified as being commonly used in hydraulic fracturing fluids.
Human Exposure and Toxity
Acetaldehyde is a metabolic intermediate in humans. It has been identified in food, beverages and cigarette smoke. By far, the main source of exposure to acetaldehyde in the general population is through metabolism of ethanol. Workers may be exposed in some manufacturing industries and during alcohol fermentation. Several isoenzymic forms of acetaldehyde dehydrogenase (ALDH) have been identified in the human liver and other tissues.
There is polymorphism for mitochondrial ALDH. Subjects that are homozygous or heterozygous for a point mutation in the mitochondrial ADLH corresponding gene have low activity of this enzyme, metabolize acetaldehyde slowly and are intolerant of ethanol alcohol. There is some metabolism of acetaldehyde in human renal tubules; the liver is the most important metabolic site. Limited studies involving human volunteers have shown that acetaldehyde was mildly irritating to the eyes and upper respiratory tract following short term exposures.
Intravenous infusion of 5% acetaldehyde at a rate of 20.6- 82.4 mg/min for up to 36 min into normal human subjects caused an increase in heart rate, ventilation and dead space, and a decrease in alveolar carbon dioxide levels. These symptoms are qualitatively and quantitatively similar to those seen after ethanol intake in subjects previously treated with disulfiram (Antabuse), a known inhibitor of ALDH. Large doses may cause death by respiratory paralysis.
Symptoms of chronic intoxication resemble those of chronic alcoholism. Acetaldehyde has been implicated as the putatively toxic metabolite in the induction of ethanol alcohol associated liver damage, facial flushing and developmental effects. Human lymphocytes (from known alcoholics) were exposed to acetaldehyde concn of 0.02 mg/mL and 0.04 mg/mL. Results indicate that chromosomal aberrations occurred at both concentrations. Acetaldehyde-DNA adducts have been observed in granulocytes and lymphocytes of human alcohol abusers.
Conclusion
Acetaldehyde: a toxic byproduct—Much of the research on alcohol metabolism has focused on an intermediate byproduct that occurs early in the breakdown process—acetaldehyde. Although acetaldehyde is short lived, usually existing in the body only for a brief time before it is further broken down into acetate, it has the potential to cause significant damage.
This is particularly evident in the liver, where the bulk of alcohol metabolism takes place . Some alcohol metabolism also occurs in other tissues, including the pancreas and the brain, causing damage to cells and tissues. Additionally, small amounts of alcohol are metabolized to acetaldehyde in the gastrointestinal tract, exposing these tissues to acetaldehyde’s damaging effects.
You may like
Related articles And Qustion
See also
Lastest Price from Acetaldehyde manufacturers

US $1.00/KG2025-04-21
- CAS:
- 75-07-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $9.00/KG2024-10-11
- CAS:
- 75-07-0
- Min. Order:
- 1KG
- Purity:
- 99.8%
- Supply Ability:
- 100tons