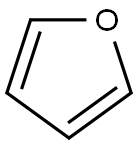
Toxicity and hazards of Furan
Furan occurs naturally in oils distilled from rosin containing pinewood. In addition, many natural foods contain the furan ring structure and substituted furans may be formed through cooking of simple carbohydrates. Furan is also found in tobacco smoke as well as wood smoke and gas emissions from gasoline and diesel engines. Furan has also been detected in industrial effluents and can be emitted to the air from petroleum refineries and coal-mining and gasification plants.
Furan is a solvent used in the organic synthesis of pyrrole, tetrahydrofuran, and thiophene. It is also used as a solvent for resins and in the production of lacquers, agricultural chemicals, and stabilizers.
Toxicity and hazards
Furan can cause eye, skin, and mucus membrane irritation, burning sensation and, in severe cases, corrosion. If inhaled, furan may produce pulmonary edema and bronchiolar necrosis. When absorbed, furan can cause central nervous system (CNS) depression to the point of narcosis and tonic seizures.
Furan is metabolized by the cytochrome P450 enzymes in the liver and other tissues. Furan is metabolized to cis-2- butene-1,4-dial. This active metabolite is acutely toxic to liver cells. The furan ring undergoes oxidative cleavage and forms highly reactive furan radical cations or epoxides, which react directly with cellular nucleophiles. These reactive metabolites may react directly with deoxyribonucleic acid (DNA) or with cellular proteins to produce disruption of cellular functions and cell death. Chronic cell death and regeneration produced by chronic furan exposure may be a significant factor in the carcinogenicity potential of the chemical. In addition, there is some evidence to suggest that the reactive metabolites of furan may induce mutations in cellular genes.
Furan may be released to the environment as a waste industrial product or from unintentional or accidental releases. If released to soil, it is expected to volatilize. If released to water, furan is not expected to adsorb to suspended particles and sediment and is likely to volatilize to ambient air. Sulfate-reducing bacteria can degrade furan. However, under nonsulfate-reducing conditions, biodegradation in soil and water is expected to be slow. In the air, furan will exist as a vapor and will be subject to degradation by reacting with hydroxyl radicals.