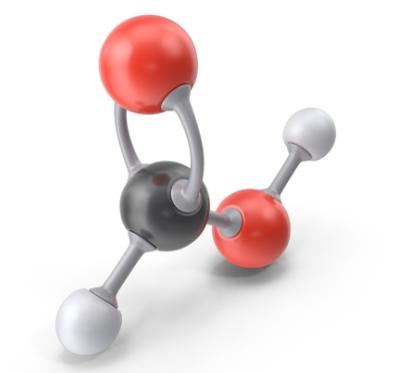
Environmental Fate of Formic acid
Formic acid is a clear, colorless liquid with a pungent odor. Formic acid was first isolated from certain ants and was named after the Latin formica, meaning ant. It is made by the action of sulfuric acid on sodium formate, which is produced from carbon monoxide and sodium hydroxide. It is also produced as a by-product in the manufacture of other chemicals such as acetic acid. It can be anticipated that use of formic acid will continuously increase as it replaces inorganic acids and has a potential role in new energy technology. Formic acid toxicity is of a special interest as the acid is the toxic metabolite of methanol.
Uses
Formic acid is principally used as a preservative and antibacterial agent in livestock feed. It is usually sprayed on animal feed or fresh hay to reduce the rate of decay and is also used as a pesticide to treat and control mites that infest honeybee hives. In the manufacturing industry, it is used as an acidulating agent for dying and finishing textiles, in tanning leather, and electroplating. Formic acid is also used as a coagulating agent for rubber latex. It is an important precursor for synthesis of chemicals and pharmaceuticals and a valuable pH regulator in resin manufacturing.
Formic acid has great potential as an in situ source of hydrogen for fuel cells, because it offers high energy density and can be handled in aqueous solution. Ag nanoparticles coated with a thin layer of Pd atoms as well as iron can significantly enhance the production of H2 from formic acid at ambient temperature. Therefore, formic acid might play a key role in the future in renewable energy technologies as a potential secondary energy vector because it would enable clean energy storage and transduction.
Environmental Fate
Formic acid is found in nature as it is produced by plants, insects, and bacteria. However, it is also used in industries for the manufacture of numerous consumer products. Therefore, the chemical may be released to the environment as a waste product or from unintentional, accidental releases. If released to soil, it is expected to biodegrade and has a short half-life. If released to water, it is expected to biodegrade and hence not likely to bioaccumulate in aquatic organisms. If released to air, it is expected to react with hydroxyl radicals contained in water vapor.
Silage as animal diet in north European countries is a source for urinary formic acid to get to the environment from animals.
In industry, exposure to formic acid can occur through the oral, dermal, and inhalation routes. Formic acid can also be produced in the mouth and stomach from ingested formaldehyde. Metabolism of methanol and formaldehyde in the liver produces formic acid, and blood and urinary concentrations might be high in severe poisoning cases.
Stings by bees, wasps, and ants may result in the subcutaneous injection of formic acid.
You may like
Related articles And Qustion
Lastest Price from Formic acid manufacturers

US $0.00/KG2025-04-21
- CAS:
- 64-18-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $10.00-500.00/kg2025-04-17
- CAS:
- 64-18-6
- Min. Order:
- 1000kg
- Purity:
- 0.98
- Supply Ability:
- 3000MT per year