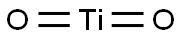Titanium dioxide: structure and uses
Titanium dioxide (TiO2) is one of the most widely investigated metal oxides in photocatalysis of renewable energy creation and environmental remediation due to its photo(electro)chemical stability and photoactivity, its nontoxicity, and low cost. TiO₂ is Rutile structured and crystallizes in the tetragonal P4₂/mnm space group. Ti⁴⁺ is bonded to six equivalent O²⁻ atoms to form an edge and corner-sharing TiO₆ octahedra mixture. The corner-sharing octahedral tilt angles are 49°. Four shorter (1.95 Å) and two longer (1.98 Å) Ti-O bond lengths exist. O²⁻ is bonded in a distorted trigonal planar geometry to three equivalent Ti⁴⁺ atoms[1].
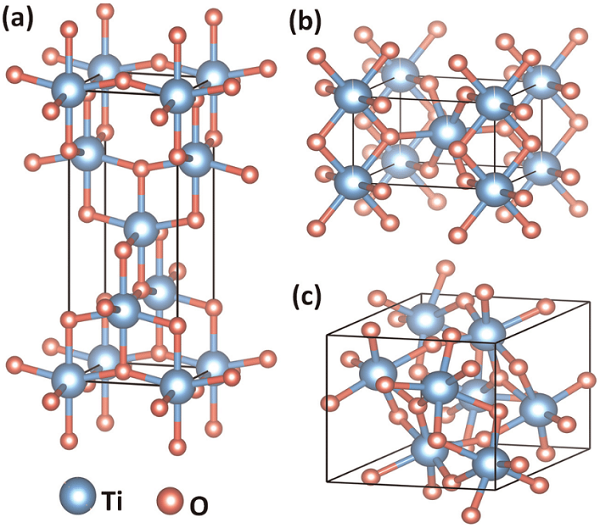
Figure 1. Crystal structures of TiO2 (a) anatase (tetragonal), (b) rutile (tetragonal), and (c) brookite (orthorhombic) polymorphs[2].
So far, TiO2 is a well-known photoelectrode material that has tremendous performance in dye-sensitized solar cell device applications. However, despite the outstanding performance, TiO2 tends to suffer from a high recombination center of electrons and holes coupled with a wide band gap (i.e., 3.2 eV). Thus, a lot of research is being carried out with the aim of enhancing device performance by reducing charge carrier recombination and improving the electrical/optical performance of TiO2 photoelectrode. In this regard, elemental doping is the most advantageous approach to modify its properties[3].
Generally, the properties and performance of TiO2 depend on its crystal phase, crystallinity, morphology, surface area, etc. Among four naturally occurring polymorphs of TiO2, i.e., anatase, Rutile, brookite, and TiO2 (B), anatase TiO2 has been acknowledged as the most active phase in photocatalysis for environmental applications. In the crystal structure of anatase, the primary TiO6 octahedron building units are significantly distorted and interconnected via corner and edge-sharing to form zigzag chains with a screw axis, which offers a relatively loose atomic stacking and low density that is correlated with its greater absorption capability and more abundant active sites than other three polymorphs, therefore, more efficient charge separation and a lower carrier recombination rate could be achieved.
The photocatalytic activity and preference of TiO2 crystals are heavily dependent upon the crystallographic orientations, so maximizing the surface of TiO2 photocatalyst to reactive facets is feasible to realize the high activity and preference of photocatalytic reactions. Increasing research attention is now being directed toward engineering the surface structure of TiO2 at the most fundamental and atomic level control of exposed active facets to optimize its physicochemical properties.
Anatase TiO2 crystals have been increasingly investigated with a high percentage of high-energy {0 0 1} and small particle size for improving photocatalytic activity. On the other hand, on the basis of the calculated results, the order for the relative photooxidation and photoreduction activities of the three surfaces of anatase TiO2 is {1 0 0} > {1 0 1} > {0 0 1}; the (1 0 1) facets have a higher activity on CO2 photoreduction than (0 0 1) facets. Moreover, it has been experimentally revealed that the true photoreactivity order of low index facets of anatase TiO2 is {0 0 1} < {1 0 1} < {0 1 0} by comparing photocatalytic hydrogen evolution, radical dotOH radical generation and photoreduction reactions from the crystals with a predominance of {0 0 1}, {1 0 1}, and {0 1 0}. Achieving TiO2 crystals with dominant low-energy {1 0 1} deserves more attention. Equally crucial to acquiring the above reactive facets for promoting surface charge carrier separation/transfer, decreasing severe bulk carrier recombination will definitely contribute to the photocatalytic activity. The most widely used strategy is to shorten the bulk diffusion length of carriers by decreasing particle size, particularly to the nanoscale. Many substantial efforts have been devoted to achieving hollow nanostructures of anatase TiO2 with high surface area by various synthetic strategies for obtaining more active sites in photocatalysis; however, most of which are usually polycrystalline and lack faceted surfaces, where a large number of grain boundaries will increase electron-hole recombination rate.
References
[1] Siddiqui, H. “Modification of Physical and Chemical Properties of Titanium Dioxide (TiO2) by Ion Implantation for Dye Sensitized Solar Cells.” Ion Beam Techniques and Applications 9 1 (2019): 0.
[2] Zhengcui Wu. “Single-crystalline titanium dioxide hollow tetragonal nanocones with large exposed (1 0 1) facets for excellent photocatalysis.” Journal of Colloid and Interface Science 490 (2017): Pages 420-429.
[3] Foo Wah Low, Sharifah Bee Abd Hamid, Chin Wei Lai. “Surface modification of reduced graphene oxide film by Ti ion implantation technique for high dye-sensitized solar cells performance.” Ceramics International 43 1 (2017): Pages 625-633.
You may like
See also
Lastest Price from TITANIUM DIOXIDE manufacturers

US $0.00-0.00/kg2025-04-09
- CAS:
- 1317-70-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000tons
US $0.00-0.00/KG2024-09-04
- CAS:
- 1317-70-0
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KG