The wide use of Xylene.
Introduction
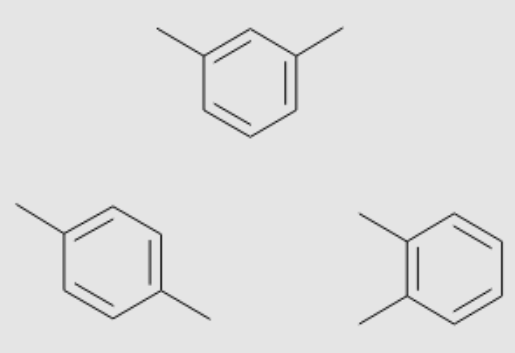
Xylene (dimethylbenzene) is a commonly used aromatic solvent with three isomeric forms: ortho, meta-, and para-xylene. Xylenes are one of the highest-volume chemicals produced and used by industry. A mixture of all three isomers is termed xylol.
Chemical property
Xylene is a colourless, flammable, liquid hydrocarbon with a characteristic aromatic odour. The odour threshold of xylenes depends on the isomer. On average, the odour threshold appears to be about 1.0 ppm. m-Xylene has an airborne odor threshold of 3.7ppm; o-xylene, 0.17 ppm; and p-xylene, 0.47 ppm.
Uses
Xylene is commonly found in varnish, ink, paint thinners, degreasers, and insecticides. Xylenes like benzene and toluene are significant components of gasoline and fuel oil. The primary uses of xylenes industrially are as solvents and synthetic intermediates. Commercial Xylene is often contaminated with other organic compounds such as ethylbenzene, toluene, benzene, trimethylbenzene, phenol, thiophene, and pyridine. The volumes of these contaminants are very minor, making up less than a fraction of 1%.
Specifically, Xylene is used as a solvent in the printing, rubber, paint and leather industries. It is found in small amounts in airplane fuel, gasoline and cigarette smoke. In dentistry, Xylene is used in histological laboratories for tissue processing, staining and coverslipping, and endodontic retreatment as a guttapercha solvent. Its high solvency factor allows maximum displacement of alcohol and renders the tissue transparent, enhancing paraffin infiltration. Its excellent dewaxing and clearing capabilities in staining procedures contribute to brilliantly stained slides[1].
Safety
Liquid Xylene is irritating to the skin and eyes, causing local vasodilation by the liberation of histamine and 5-hydroxytryptamine. Direct xylene eye splash causes initial transient discomfort and hyperemia of the conjunctiva. The cornea may rapidly develop a visually significant central haze, usually limited to the anterior portions of the stroma. A characteristic clinical feature reveals clear vacuoles resembling microcystic corneal epithelial edema but localized mainly in the anterior stroma. The stromal vacuoles are characteristic and only mentioned in a few other entities, such as n-butanol and nitronaphthalene exposure. In contrast, epithelial cystic changes differ from stromal vacuoles and are commonly seen in other keratopathies, such as contact lens toxicity and increased intraocular pressure.
The toxicokinetics (TK) and acute toxicity of toluene, xylenes, and other aromatic solvents are similar. Xylenes and the others are well absorbed from the lungs and gastrointestinal tract, distributed to tissues according to tissue blood flow and lipid content, exhaled to some extent, well metabolized by hepatic P-450s, and largely excreted as urinary metabolites.
References:
[1] REENA KANDYALA S T R Sumanth Phani C Raghavendra. Xylene: An overview of its health hazards and preventive measures.[J]. Journal of Oral and Maxillofacial Pathology, 2010, 14 1. DOI:10.4103/0973-029X.64299.You may like
Related articles And Qustion
Lastest Price from Xylene manufacturers
US $0.00/kg2025-04-15
- CAS:
- 1330-20-7
- Min. Order:
- 20kg
- Purity:
- 99.0%
- Supply Ability:
- 20 tons

US $1.00/g2024-09-29
- CAS:
- 1330-20-7
- Min. Order:
- 1g
- Purity:
- 99
- Supply Ability:
- 20tons