The wide range of applications of Sodium chlorite
Description
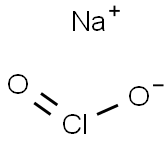
Sodium chlorite (NaClO2) is also called chlorous acid, sodium salt textone, and Miracle Mineral Solution. It is composed of sodium (Na), chlorine (Cl), and oxygen (O2).

Synthetic method
Sodium chlorite is manufactured in two ways: electrolysis and reduction. The electrolysis method is characterized by high investment and complicated operation, while the reduction method is used in most plants to produce sodium chlorite. Production of sodium chlorite with a reducing agent consists of two steps: (1) preparation of chlorine dioxide by reacting sodium chlorate in sulfuric acid, and then (2) reaction of chlorine dioxide with a reducing agent in sodium hydroxide solution[1]. The first step is the key to determining product economics and production technology.
Uses
Sodium chlorite is a highly efficient bleaching agent and oxidative disinfectant, widely applied in textile, fiber, pulp, and paper bleaching. It is characteristic of lower harm to fiber. It also whitens sugar, starch, grease, ointment, and wax. When used in purifying water, little of the remaining chlorine would be found. It is also used extensively in sterilization, and deodorization in sewage treatment processes. Since reaction conditions to produce chlorine dioxide from sodium chlorite are mild, sodium chlorite is the primary feedstock chemical for producing chlorine dioxide in mini-type generators[2].
In organic synthesis, NaClO2 is an inexpensive and versatile reagent, which has been explored for preparations of carboxylic acids from aldehydes or primary alcohols, amides from imines, enones from allylic and benzylic substrates, epoxides from olefins and g-hydroxybutenolides from furans. Moreover, this reagent has also been employed in the oxidative deprotection of 1,3-dithiane groups, as an oxidant and hydroxyl ion pump in osmiumcatalyzed asymmetric dihydroxylation as well as in the iodination of aromatic and heterocyclic compounds.
Sodium chlorite could enhance methane production from the anaerobic digestion of hybrid Pennisetum by selectively removing lignin. SCA pretreatment can selectively remove lignin with minimal impact on cellulose and hemicellulose. After up to 200 minutes of SCA treatment, 79.4% of lignin was removed, and over 90% of the holocellulose was retained. Lignin removal significantly enhanced the biodegradability from 59.6% to 86.4% and increased methane production by 38.3%[3]. Energy balance showed that SCA pretreatment efficiently increased the energy output of hybrid Pennisetum.
References
[1] Yu Qian. “A clean production process of sodium chlorite from sodium chlorate.” Journal of Cleaner Production 15 10 (2007): Pages 920-926.
[2] Marcelle de Ferreira. “Sodium Chlorite.” Synlett 5 (2011): 730-731.
[3] Xihui Kang. “Enhanced methane production from anaerobic digestion of hybrid Pennisetum by selectively removing lignin with sodium chlorite.” Bioresource Technology 295 1 (2019): 122289.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium chlorite manufacturers
US $658.00-585.00/ton2025-03-07
- CAS:
- 7758-19-2
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 5000ton

US $0.00/KG2025-03-05
- CAS:
- 7758-19-2
- Min. Order:
- 1KG
- Purity:
- 80
- Supply Ability:
- 200 MT