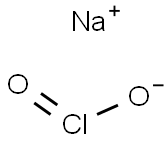
Health and safety of sodium chlorite
Introduction
Sodium chlorite is commercially available in two different physical forms, either as a dry, flake material or in an aqueous solution. Unlike most chemicals, some of the hazards associated with this chemical may differ, depending on the form of this product.
Dry sodium chlorite, which is a white flake product with a slight chlorine odor, is an 80% active product. Sodium chlorite in its dry form is a strong oxidizer.
Sodium chlorite solutions are clear to slightly yellow in appearance, have a slight chlorine odor, and are available in different grades and concentrations ranging from 7.5% to 37% by weight. The most widely used solution products contain 25% active sodium chlorite. Sodium chlorite solutions are classified as corrosive. The hazards and precautions specific to each form of this product will be discussed separately, below, followed by information common to both forms.

All users should read the appropriate product label and Material Safety Data Sheet (MSDS) before handling either form of sodium chlorite.
Dry Sodium Chlorite
Dry sodium chlorite may cause irritation or burns to the skin and eyes. It is harmful if swallowed.
Chemical Hazards
Sodium chlorite in its dry form is a strong oxidizer. An oxidizer is a compound that initiates or promotes combustion in other materials. This means that if sodium chlorite comes into contact with combustible materials, it can react rapidly and ignite. However, sodium chlorite will not normally burn by itself. Examples of combustible materials are oil or grease (such as from a forklift), wood (such as pallets), leather, cloth, paints, organics, and in some cases dirt. It is important to keep these materials away from dry sodium chlorite.
Dry sodium chlorite can be explosive in contact with chlorine, acids or acid materials such as alum. Contamination by these materials may start a chemical reaction, causing generation of heat and emission of chlorine dioxide, a poisonous and potentially explosive gas.
Handling Precautions
Do not get in eyes, on skin or on clothing. Do not taste or swallow. Avoid breathing dust and fumes. Keep containers closed. Do not handle with bare hands. Use only clean, dry utensils when handling. Mix only into water. Never add water to product. Remove and wash contaminated clothing to avoid fire.

Sodium Chlorite Solution
Sodium chlorite solutions are corrosive and cause skin and eye irritation or burns. It is harmful if swallowed.
Chemical Hazards
Sodium chlorite solution becomes a fire or explosion hazard if allowed to dry and can ignite on contact with combustible material.
As with the dry product, sodium chlorite solutions are incompatible with materials such as organics, oxidizers, reducing agents, soap products, acids, paint products, combustible materials, and in some cases, dirt. Contamination may start a chemical reaction with generation of heat, liberation of hazardous gases (chlorine dioxide a poisonous, explosive gas), and possible fire and explosion.
Handling Precautions
Do not get in eyes, on skin, or on clothing. Do not taste or swallow. Avoid breathing fumes, mists or aerosols. Do not handle with bare hands. Mix only into water. This product becomes a fire hazard if allowed to dry. Remove and wash contaminated clothing to avoid fire.
Potential Health Effects
The health effects discussed below are common to both the dry and solution forms of sodium chlorite.
Eye Contact
Direct contact, or exposure to dust, mists or fumes may cause severe irritation and possibly burns. Symptoms may include tearing, redness and in severe cases, eye damage due to burns.
Skin Contact
Direct contact may cause severe irritation and/or burns with symptoms of redness, itching, swelling and possible destruction of tissue.
Ingestion
Ingestion may cause gastroenteritis with any or all of the following symptoms: nausea, vomiting, lethargy, diarrhea, bleeding or ulceration. Acute ingestion of large quantities may also cause anemia due to the oxidizing effects of the chemical.
Inhalation
Inhalation of dusts, vapors or mists may cause irritation of the mucous membranes and respiratory tract. Symptoms may include coughing, bloody nose, and sneezing. Severe overexposures may cause lung damage.
Notes to Physician: Chlorine dioxide vapors are emitted when this product contacts acids or chlorine. If these vapors are inhaled, monitor patient closely for delayed development of pulmonary edema which may occur up to 48-72 hours post-inhalation. Following ingestion, neutralization and use of activated charcoal is not indicated.
Traumatic Shock: Whenever injured persons are being cared for, the person administering first aid should watch for signs of traumatic shock. Traumatic shock may follow serious injury and is a depressed condition of many of the body functions due to inadequate blood circulation throughout most of the body. Signs of shock are pale, moist cool skin; shallow and irregular breathing; and weak pulse. Beads of perspiration may be noted about the lips, forehead, palms, and armpits. The patient may become nauseated. To treat shock, keep the patient lying down and as warm and comfortable as possible. Raise the patient’s feet eight to twelve inches unless there is head injury, breathing difficulty, or if the patient complains of added pain.
Safety
Educating Employees Only trained and properly protected personnel should be allowed to enter areas where sodium chlorite is present. Before working with or handling this product, the user should be trained and familiar with the proper safety precautions to follow. Additionally, all users and area supervisors should be trained in first aid procedures in case of accidental exposures to sodium chlorite.
Safe Work Practices
Water should always be easily accessible wherever sodium chlorite is stored or used. Safety showers and eyewash fountains should be close by and clearly marked. Portable or temporary systems are available for remote areas. Every precaution should be taken to ensure that a suitable system is in place and operational before handling sodium chlorite.
Good Housekeeping
Good housekeeping practices are important where sodium chlorite is used. All spills should be contained and immediately recovered or flushed with water into a chemical sewer or a segregated holding tank or pond provided for the specific purpose of neutralization. Sodium chlorite must never be flushed to a sanitary sewer or other outlet connecting to waterways or uncontrolled runoff streams. Contact local and federal authorities for applicable regulations
You may like
Related articles And Qustion
Lastest Price from Sodium chlorite manufacturers
US $658.00-585.00/ton2025-03-07
- CAS:
- 7758-19-2
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 5000ton

US $0.00/KG2025-03-05
- CAS:
- 7758-19-2
- Min. Order:
- 1KG
- Purity:
- 80
- Supply Ability:
- 200 MT