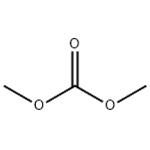
The wide range of applications: Dimethyl carbonate
Description
Dimethyl carbonate is a versatile compound that represents an attractive, eco-friendly alternative to both methyl halides (or dimethyl sulfate) and phosgene for methylation and carbonylation processes, respectively.

Synthesis method
Dimethyl carbonate (DMC) is a green compound with a wide variety of applications. To date, many routes have been applied to synthesize DMC, such as the phosgene route, transesterification route, urea alcoholysis route, methanol oxidative carbonylation route, and direct synthesis route from methanol and carbon dioxide (CO2). Among these routes, phosgenation, transesterification, and liquid-phase methanol oxidative carbonylation routes have achieved industrialization. Notably, the use of the phosgenation process has been discontinued due to the use of hypertoxic raw material, phosgene. Transesterification and liquid-phase methanol oxidative carbonylation routes are used intensively in DMC's large-scale industrial production. Nonetheless, these two routes also display some disadvantages. For example, the transesterification route suffers from high costs and large amounts of wastewater and residue. In the liquid-phase methanol oxidative carbonylation route, water formed during the reaction severely shortens the catalyst's lifetime.
Recently, CO2-based routes to produce DMC have attracted much attention due to the environmental benefits of CO2 utilization. The DMC production contains the processes of urea synthesis, propylene carbonate (PC) synthesis, DMC synthesis, and DMC/methanol azeotrope separation. The methods of urea synthesis, DMC synthesis, and DMC/methanol azeotrope separation were well-developed [1].
Uses
Dimethyl carbonate (DMC), an important and environmentally friendly chemical intermediate, has displayed a wide range of applications in the industrial field in recent decades[2]. Polycarbonate (PC), mainly derived from DMC, is extensively utilized in the building, automobile, and medical device industries. DMC is also an electrolyte solvent in lithium batteries due to its high dielectric constant. Given its high oxygen content (53%) and octane number (105), good gasoline/water distribution coefficient, low toxicity, and rapid biodegradability, DMC has been regarded as a potential additive to fuel oil. The proper amount of DMC added to diesel can decrease the soot particle emission of engines and consequently alleviate environmental pollution. DMC with methyl and carbonyl groups, as an alternative to toxic dimethyl sulfate and phosgene, can also be used as an environmentally friendly reagent for methylation and carbonylation, respectively.
Methylation
In fact, the reactivity of DMC is tunable: at T = 90 °C, methoxycarbonylations take place, whereas, at higher reaction temperatures, methylation reactions are observed with a variety of nucleophiles. In the particular case of substrates susceptible to multiple alkylations (e.g., CH2-active compounds and primary amines), DMC allows unprecedented selectivity toward mono-C- and mono-N-methylation reactions. DMC-mediated methylations are catalytic reactions that use safe solids (alkaline carbonates or zeolites), thereby avoiding the formation of undesirable inorganic salts as byproducts. The reactivity of other carbonates is reported as well: higher homologs of DMC (i.e., diethyl and dibenzyl carbonate) are excellent mono-C- and mono-N-alkylating agents, whereas asymmetrical methyl alkyl carbonates (ROCO2Me with R ≥ C3) undergo methylation processes with chemoselectivity up to 99%[3].
References
[1] Shi, Li et al. “Plant-wide process design of producing dimethyl carbonate by indirect alcoholysis of urea.”Computer Aided Chemical Engineering 44 (2018): 115-120.
[2] Wenjie Deng . “A review on transesterification of propylene carbonate and methanol for dimethyl carbonate synthesis.” Carbon Resources Conversion 2 3 (2019): Pages 198-212.
[3] P. Tundo, M. Selva. “The Chemistry of Dimethyl Carbonate.” ChemInform 10 1 (2002).
You may like
Related articles And Qustion
See also
Lastest Price from Dimethyl carbonate manufacturers
US $0.00/kg2025-04-15
- CAS:
- 616-38-6
- Min. Order:
- 20kg
- Purity:
- 99.0%
- Supply Ability:
- 20 tons

US $1.00/KG2025-03-07
- CAS:
- 616-38-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt