The Way Things Work: Thrombin
Thrombin is the principal enzyme of hemostasis. It catalyzes the conversion of fibrinogen to fibrin and activates procoagulant factors V, VIII, XI, and XIII. Additionally, when bound to thrombomodulin, it activates protein C, an anticoagulant zymogen. Thrombin also activates platelets, regulates endothelial cell function, and has a host of direct actions on other cells. In addition to its catalytic site, thrombin contains a Na+-binding site and two anion-binding exosites, ABE1, which binds fibrinogen and thrombomodulin, and ABE2. Thrombin also contains an insertion site for its newly formed N-terminus that develops following proteolytic cleavage of prothrombin. The insertion produces an internal salt bridge that results in reorganization and activation of the catalytic site.
Thrombin begins life as an inactive zymogen, prothrombin. The conversion of prothrombin to thrombin is catalyzed by factor Xa and includes two essential proteolytic cleavages, producing three intermediates: prethrombin 2, meizothrombin, and Fragment 1.2 (F1.2), which binds ABE2. Since the identification of these intermediates nearly 40 years ago,1 the mechanism(s) underlying the development of the enymatic activity of thrombin and the remarkable differences in specificity of thrombin and other prothrombin derivatives have been the subject of intense investigation. More than 100 x-ray structures have been reported in hundreds of papers describing the functional properties of thrombin and other prothrombin derivatives. Now Kamath et al. from Krishnaswamy’s lab at Children’s Hospital of Philadelphia propose a novel mechanism by which thrombin activation and specificity is explained by a continuum of states from the enzymatically inactive zymogen, prothrombin, to catalytically active thrombin (Figure).
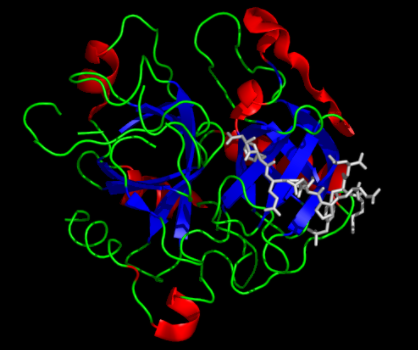
Using isothermal titration calorimetry to provide very accurate estimates of binding constants, the authors measured binding of F1.2 to four prothrombin derivatives that represent putative structural intermediates along the continuum from the zymogen, prothrombin, to thrombin. Binding to the derivatives increased with increasing zymogenicity: S195A prethrombin 2 > TAT thrombin > S195A thrombin > FPR thrombin. Additionally, binding of F1.2 to S195A thrombin increased with progressively decreasing concentrations of Na+, consistent with conversion of S195A thrombin to a more zymogen-like state. In contrast, the binding of the thrombin inhibitor DAPA into the catalytic site of zymogen-like TAT thrombin decreased the binding of F1.2, consistent with conversion to an enzyme-like state.
The voluminous thrombin literature is dominated by a model in which binding of Na+ to thrombin activates its prohemostatic functions but not its anticoagulant function.2 The model predicts that significant amounts of both the Na+-free and Na+- bound forms of thrombin are present following prothrombin activation in vivo. It also predicts that hyponatremia and hypernatremia are risk factors for bleeding and thrombosis, respectively, by favoring the anticoagulant Na+-free and procoagulant Na+-bound forms of thrombin. However, by measuring the kinetics of peptidyl substrate hydrolysis by thrombin as a function of Na+ concentration, Kamath et al. estimated that the dissociation constant for Na+ binding to thrombin at physiological pH and temperature is only 34 mM. Because the normal concentration of extracellular Na+ is ~140 mM, this indicates that thrombin is saturated with Na+ under physiological and pathological conditions, which is inconsistent with the prediction of the Na+-activated thrombin model. Additionally, the continuum model fundamentally differs from the Na+-activated thrombin model, because it predicts that Na+-free thrombin is zymogen-like and is not an “anticoagulant” enzymatically active thrombin. The continuum model also predicts that binding of F12 to ABE2, which favors the zymogen-like state, will oppose binding of thrombomodulin and fibrinogen to ABE1 and vice versa. The authors propose that further application of isothermal titration calorimetry to study simultaneous binding ligands to ABE1 and ABE2 will provide additional testing of their model.
Kamath et al. provide evidence to support a novel mechanism to explain how thrombin functions. The fundamental feature of the model is ligand-dependent shuttling of prothrombin and thrombin along a single trajectory from zymogen-like to enzyme-like states. The specificity of thrombin toward prohemostatic and anticoagulant substrates is accounted for simply by competition between macromolecular ligands: F1.2 bound to ABE2 in prothrombin favors the zymogen-like state, whereas fibrinogen or thrombomodulin bound to ABE1 favor the enzyme-like state. The model contrasts sharply with an alternative model in which Na+ is the major driving force behind the prohemostatic functions of thrombin.2 Further testing of these models has fundamental implications for understanding the regulation of hemostasis and more generally how ligation states regulate the function of the enzymes. Additionally, it potentially will allow a better understanding of antithrombotics that target thrombin and provide strategies for developing new antithrombotics.
1. Esmon CT, Owen WG, Jackson CM. The conversion of prothrombin to thrombin. II. Differentiation between thrombin- and factor Xa-catalyzed proteolyses. J Biol Chem. 1974;249:606-611.
2. Di Cera E. Thrombin. Mol Aspects Med. 2008;29:203-254.
You may like
Lastest Price from Thrombin manufacturers

US $0.00/KG2025-04-21
- CAS:
- 9002-04-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20kg/month

US $0.00/kg2025-04-02
- CAS:
- 9002-04-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000kg