The uses of trans-4-Hydroxy-D-proline
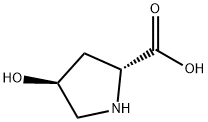
Trans-4-hydroxy-D-proline is a 4-hydroxy-D-proline in which the hydroxy group at position 4 has S-configuration.
The method was essentially similar to that employed by Neuberger for the conversion of hydroxy-L-proline to allohydroxy-L-proline, and the yields, for the most part, were much the same. A few modifications and a brief description of the intermediate compounds are as follows. Treatment of acetylallohydroxy-D-proline with diazomethane led to an apparently noncrystallizable oil. The latter was forthwith treated with purified p-toluenesulfonyl chloride in dry pyridine solution, yielding crystalline N-acetyl-O-toluenesulfonylallohydroxy-D-proline methyl ester. After recrystallization from an ether-alcohol mixture (2:1), the product melted at 143.5℃ (corrected); [α]D for a 1 per cent solution in ethanol at 25℃ = +32.0℃. Calculated C 52.7, H 6.1, N 4.1; found C 52.2, H 6.0, N 4.3.
The ester was dissolved in 5 times its weight of warm methanol, the solution chilled, and the resulting crystalline suspension shaken at 5℃ for 18 hours with the calculated amount of 1 N NaOH. Neutralization of the clear solution with an equal volume of 1 N HCl yielded an oil, which was brought to crystallization by repeated solution in ethyl acetate and precipitation with petroleum ether. The compound, which was N-acetyl-O-toluenesulfonylallohydroxy-D-proline, possessed a melting point of 143.5℃, or exactly that of the ester in the preceding step. A mixture of the two melted over a range of 118-120℃.
A further means of distinguishing the two compounds was the complete insolubility of the ester in cold 1 N NaOH, in contrast to the rapid and complete solubility of the acid in this solvent. The specific rotation of the compound was +30.5℃. Calculated C 51.4, H 5.3, N 4.3; found C 51.0, H 5.3, N 4.3.
The N-acetyl-O-toluenesulfonylallohydroxy-n-proline was heated at 100℃ for 20 minutes with 2 equivalents of 0.5 N NaOH, and after being cooled, an equal volume of 0.5 N HCl was added. The solution was evaporated to dryness in vacua and extracted several times with hot acetone. The combined and filtered acetone extractswere evaporated to a thick, yellow syrup, which was a mixture of acetylhydroxy-n-proline and p-toluenesulfonic acid. The syrup was taken up in 2 N HCl and refluxed for 2 hours. At the end of this period, the solution was decolorized with norit, and the filtrate evaporated in vucuo to dryness. The residue was dissolved in water and treated successively in the usual way with excess silver carbonate and hydrogen sulfide.
The final, clear filtrate was evaporated to dryness in vacua, the syrupy residue taken up in the minimum amount of water, and the resulting solution treated with redistilled aniline to pH 5 to 6. On addition of ethanol to 80 per cent, crystalline hydroxy-d-proline was obtained[1].
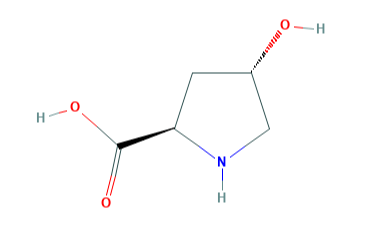
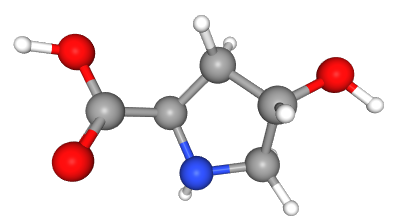
Fig 1. Chemical structure formula and three-dimensional structure of trans-4-Hydroxy-D-proline
Trans-4-hydroxy-D-proline can also prepared by N-(3,5-dinitrobenzoyl)-(4R)-hydroxy-L-proline,trans-1-(3,5-dinitro-benzoyl)-4-hydroxy-D-proline,trans-4-hydroxy-D,L-proline and etc.
References
[1.]Journal of Biological Chemistry, , vol. 204, p. 307,313.
[2.]Journal of Biological Chemistry, , vol. 195, p. 383,384.
[3.]Acta Physica et Chemica, , vol. 3, p. 118.
[4.]Bulletin de la Societe Chimique de France, , p. 1015.
You may like
See also
Lastest Price from trans-4-Hydroxy-D-proline manufacturers
US $0.00-0.00/kg2025-04-04
- CAS:
- 3398-22-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton
US $1.00/KG2024-07-20
- CAS:
- 3398-22-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20T
![904316-92-3 3-Oxa-8-azabicyclo [3.2.1] octane, hydrochloride (1:1); Synthesis; Synthetic intermediate](httpss://www.chemicalbook.com/NewsImg/2020-1-9/20201910572510598.jpg)
