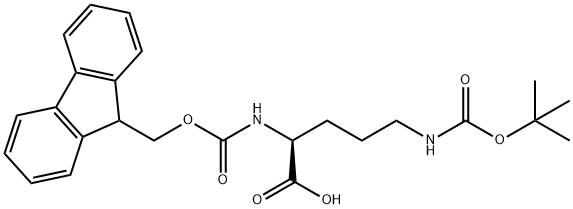
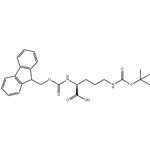
The uses of Fmoc-Orn(Boc)-OH
Introduction
Ornithine is a nonprotein amino acid from diet, endogenous arginine, and the first step of creatine synthesis. It is the substrate for ornithine-δ-aminotransferase (OAT, 10q26), which catalyzes proline synthesis. Fmoc-Orn(Boc)-OH is an ornithine-containing amino acid building block. It has been used to conjugate ornithine to GFP-labeled peptides, enhancing cell permeability compared to unconjugated GFP-labeled peptides. This compound helps prepare cyclic peptides and special arginine derivates.
Synthesis method
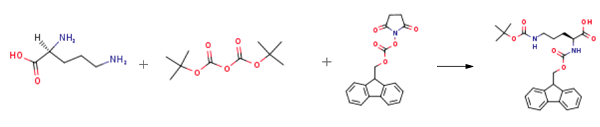
2 mmol L-Orn was weighted and dissolved in 15 ml of acetonitrile; 16 mmol Boc2O was dissolved in acetonitrile and added dropwise to an acetonitrile solution of ornithine to react and generate a complex; 10 ml 20% sodium bicarbonate aqueous solution was added, and 2 g of anhydrous sodium carbonate and an appropriate amount of 8-hydroxyquinoline were added in batches; the mixture was stirred and reacted at room temperature for 4 hours to remove copper ions in the complex; then 2 mmol Fmoc-OSu was added to the solution above, followed by stirring at room temperature for 2 hours; the resultant was recrystallized with an ethyl acetate/petroleum ether mixed solvent, to obtain a peptide head intermediate Fmoc-L-Orn (Boc)-OH[1].
Uses
Fmoc-Orn(Boc)-OH is an important material that can be used as a building block for synthesizing many organic compounds[2].
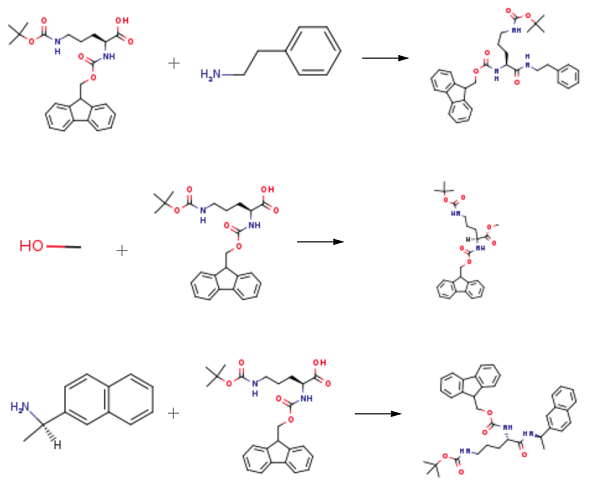
This compound could be used to synthesize (9H-fluoren-9-yl)methyl tert-butyl (5-oxo-5-(phenethylamino)pentane-1,4-diyl)(S)-dicarbamate. To a solution of Fmoc-Orn(Boc)-OH (1 eq., 4 g) in DCM (40 mL) were added BOP (1.1 eq., 4.28 g) and NMM (1.2 eq., 713 μ) and the mixture was stirred at room temperature for 10 minutes, before adding phenethylamine (1.2 eq., 1.06 mL). The reaction mixture was stirred under argon at room temperature (rt) overnight. The resulting slurry was filtered and dried under a vacuum. The resulting white powder was solubilized in EtOAc and successfully washed with aqueous hydrochloric acid (IN), saturated aqueous sodium bicarbonate, and brine and dried over sodium sulfate. The solution was filtered and concentrated under a vacuum. No further purification step was performed, and the desired compound was obtained as a white powder with a 79% yield (3.87 g).
Fmoc-Ornithinol(Boc)-OH (909 mg, 454.5 g/mol, 2.0 mmol, 1.0 eq.) was dissolved in methanol (28 ml). DCC (495 mg, 206.33 g/mol, 2.4 mmol, 1.2 eq.) and DMAP (24 mg, 122.17 g/mol, 0.2 mmol, 0.2 eq.) were added,. The reaction mixture was stirred overnight at room temperature before evaporating to dryness. The residue was dissolved in DCM. The formed precipitate was filtered off, and the filtrate was evaporated to dryness[3]. The reaction product was purified by silica gel chromatography to obtain 480 mg (53 % yield) of methyl (S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-5-((tert-butoxycarbonyl)amino)pentanoate.
Fmoc-Orn (Boc) -OH (100.2 mg, 454.52 g/mol, 0.22 mmol, 1 eq), DIC (34. 4 µl, 126.20 G/MOL, 0.806 G/CM3, 0.22 MMOL, 1 eq) and HOBt (29.7 mg, 135.12 g/mol, 0.22 mmol, 1 eq) were dissolved in dry DMF/DCM (1/1,4 ml). After 10 minutes, (R)-1-(2-NAPHTHYL), ETHYLAMINE (37.7 mg, 171.24 g/mol, 0.22 mmol, 1 eq, Acros) was added to the reaction mixture. After overnight stirring, the temperature was raised to 40°C and kept there for 2 hours. The solvent was then evaporated, and the residue was purified with flash chromatography. 5- (N-BOC-AMINO)- (S)-2-(N-FMOC-AMINO)-N-((R)-1-(2-NAPHTHYL) ETHYL) pentanamide was obtained with quantitative yield[4].
References
[1] Current Patent Assignee: DALIAN NATIONALITIES UNIVERSITY - US2017/355667, 2017, A1. Location in patent: Paragraph 0059; 0069; 0073; 0085.
[2] Current Patent Assignee: CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE - WO2013/178810, 2013, A1. Location in patent: Page/Page column 30.
[3] Current Patent Assignee: WURSTER - WO2006/123020, 2006, A1. Location in patent: Page/Page column 77.
[4] Current Patent Assignee: WURSTER - WO2005/33069, 2005, A1. Location in patent: Page/Page column 25
You may like
See also
Lastest Price from Fmoc-Orn(Boc)-OH manufacturers
US $0.00-0.00/kg2025-04-21
- CAS:
- 109425-55-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1T+