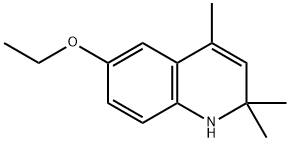
The uses of Ethoxyquin in food
Introduction
Pure ethoxyquin (EQ; 6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline; CAS number 91-53-2) is a light yellow liquid, changing colour to brown if exposed to oxygen. It also tends to polymerize on exposure to light and air. The scent of EQ is described as mercaptan-like. As a nonpolar substance, EQ is soluble only in organic solvents.
Development

Ethoxyquin is also known as Santoquin, Santoflex, and Quinol. It was originally developed in the rubber industry to prevent rubber from cracking due to the oxidation of isoprene. The Monsanto Company (USA), taking into account its high antioxidant efficiency and stability as well as low costs of synthesis, refined it later for use as a preservative in animal feeds because it protects against lipid peroxidation and stabilizes fat-soluble vitamins (A, E). Presently, ethoxyquin is used primarily as an antioxidant in canned pet food and in feed intended for farmed fish or poultry[1].
Uses
Ethoxyquin is a synthetic antioxidant that is included in some animal and human foods as a preservative to protect fats and fat-soluble vitamins from oxidative degradation[2]. Without antioxidants in pet foods, oxidative processes lead to rancidity of the food. Rancid fat is offensive in odour and flavour and includes compounds that are toxic when consumed. Including antioxidants in commercial pet foods ensures the food’s safety, nutritional integrity, and flavour. Starting in the late 1980s ethoxyquin was identified by dog breeders and owners as a potentially dangerous synthetic preservative. Depending on the source of information, ethoxyquin was believed to be responsible for reproductive problems, autoimmune disorders, behaviour problems, and various types of cancers in dogs and cats.
Genotoxicity
Ethoxyquin is not mutagenic to Salmonella TA100 and TA98. Ethoxyquin induced DNA damage in human lymphocytes in a dose-dependent manner; the DNA fragmentation induced by ethoxyquin in the presence of the metabolic activation system was always significantly lower compared to cells treated with the same doses of ethoxyquin alone[3]. Ethoxyquin-induced DNA damage is caused by the free radical generated. In vivo, ethoxyquin gave a weak positive response in the liver micronucleus test in juvenile rats but negative responses in the mouse bone marrow micronucleus test and the UDS test using rat liver. Although ethoxyquin and/or its metabolite(s) induce chromosomal aberration, the influence on the chromosomal aberration is likely to be associated with ethoxyquin’s action on the functional protein component rather than the direct DNA damage.
References
[1] A. Blaszczyk, J. Skolimowski, A. Augustyniak. “Ethoxyquin: An Antioxidant Used in Animal Feed.” International Journal of Food Science 26 1 (2013).
[2] on Additives, EFSA Panel and Products or Substances used in Animal Feed (FEEDAP). “Safety and efficacy of a feed additive consisting of ethoxyquin (6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline) for all animal species (FEFANA asbl).” EFSA Journal 20 3 (2022).
[3] Yamazoe, Y. and K. Mitsumori. “Assessment of Nongenotoxic Mechanisms in Carcinogenicity Test of Chemicals; Quinone, Quinone Imine, and Quinone Methide as Examples." Thresholds of Genotoxic Carcinogens (2016): 171-192.
You may like
See also
Lastest Price from Ethoxyquin manufacturers

US $10.70/kg2025-06-10
- CAS:
- 91-53-2
- Min. Order:
- 10kg
- Purity:
- 95%
- Supply Ability:
- 10000kg

US $1.00/PCS2025-04-21
- CAS:
- 91-53-2
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 10 mt