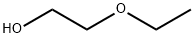The uses and toxicity of 2-Ethoxyethanol
2-Ethoxyethanol is a stable, colorless, flammable liquid, synthetically produced throughout the world. It belongs to a larger group of glycol ether solvents. 2-Ethoxyethanol is commercially referred to as Ethyl Cellosolve or Cellosolve, a trademark registered by Union Carbide in 1924. It was first synthesized to have the same chemical properties of both alcohols and ethers (hydrophilic and lipophilic) but less volatile, which improves production characteristics. The glycol ethers are made by reacting anhydrous alcohols with ethylene oxide.

Uses
2-Ethoxyethanol is widely used as an industrial solvent and production intermediate. It is produced by the reaction of ethylene oxide with ethanol. The glycol ethers are miscible in polar and nonpolar solutions, which make them useful solvents in paints and surface coatings, stains, lacquers, inks, and dyes. Additional uses include industrial deicing, hydraulic fluids, and cleaning agents. 2-Ethoxyethanol was once used in cosmetic products but is no longer used due to toxicity associated with dermal absorption. Global production has been on the decline in recent years based on demonstrated toxicity through oral, dermal, and inhalation routes of exposure. The use of ethylene glycol ethers has largely been replaced by relatively safer substitutes, primarily propylene glycol ethers; however, their use as a solvent and chemical process intermediate poses potential for release into the environment.
Mechanism of Toxicity
The toxicity associated with 2-ethoxyethanol is likely caused more by the primary metabolite, ethoxyacetic acid, than by the parent compound. The metabolites have a longer half-life implying a higher accumulation following repeated exposures. Both in vitro and in vivo studies have shown toxic effects from administration of the metabolites that were not seen at higher doses of the parent. Developmental and male reproductive toxicity has been widely documented for several compounds in the glycol ether family, and potency is associated with the length of the hydrocarbon chain: the shorter the chain, the more potent the developmental and reproductive effects. Despite the vast collection of toxicity studies conducted internationally, the exact mechanism of developmental and reproductive toxicity is not well understood. A potential mechanism for the male reproductive toxicity is direct action on Sertoli and/or germ cells by ethoxyacetic acid. The testes have relatively high levels of cytochrome P450 and are an active site of metabolism. Investigators have found that ethoxyacetic acid can cause degeneration of spermatocytes in vitro, and damage to spermatocytes seen in vivo can be suppressed when metabolism of 2-ethoxyethanol is inhibited.
You may like
Lastest Price from 2-Ethoxyethanol manufacturers

US $0.00/kg2025-06-23
- CAS:
- 110-80-5
- Min. Order:
- 1000kg
- Purity:
- i
- Supply Ability:
- 80tons

US $10.00/KG2025-04-21
- CAS:
- 110-80-5
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt