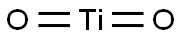The uses and safety of Titanium dioxide
Description
Titanium dioxide (TiO2) is one of the most widely used metal dioxides for applications such as semiconductors, photocatalysts, pigments, pharmaceutical excipients, and antimicrobials[1].

Uses
Titanium dioxide is a pigment widely used worldwide for a variety of applications. Much of the TiO2 produced is employed in paints and coatings, followed by plastic products and pulp and paper. It is also extensively employed in photovoltaic systems to boost the performance of dye-based cells[2]. Non-pigment TiO2 is approved for cosmetics in the United States Pharmacopeia and the EU Cosmetic Products Regulation. Last but not least, TiO2 is authorized in pigment form as an excipient in food and many medical products. In the USA, it is approved at the maximum concentration of 1% (w/w), and in Europe, it is approved at quantum satis levels as a color additive E171.
Sunscreen
Titanium dioxide is used in mineral-based sunscreens to help reflect and scatter UVA and UVB rays. Because titanium dioxide can help prevent sunburn and sun damage, SPF products with this ingredient can generally be used by all skin types looking to protect their skin from the sun and unlike chemical sunscreen, formulated with ingredients to absorb damaging sun rays, titanium dioxide sunscreen works by forming a protective barrier on your skin.
Toothpaste
TiO2 is also incorporated in toothpaste because it affects enamel and cementum solubility. Although Ti solutions at high concentrations have a strong acidity and might damage the tooth surface, low-concentration solutions combined with highly concentrated fluorides decrease the solubility of cementum. Titanium binds to the organic molecules of the dental tissue. In this way, fluorides are trapped in the matrix, making the surface less soluble and allowing a slower release of fluorides over time. Cementum is more beneficiated by this effect since it has a more excellent organic component than enamel.
Safety
Laboratory and animal studies have shown that TiO2 NPs can cause oxidative damage and lipid peroxidation in different cell types, potentially resulting in respiratory, cardiovascular, and nervous system dysfunction. The International Agency for Research on Cancer (IARC) and the IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (ECHA) have categorized inhaled TiO2 as possibly carcinogenic to humans[3].
References
[1] “Surface Modification of Titanium Dioxide Microparticles Under Ultrasonic Treatment.” International Journal of Pharmaceutical Research 14 1 (2020).
[2] Sima Hashemi Nemati, Afra Hadjizadeh. “Gentamicin-Eluting Titanium Dioxide Nanotubes Grown on the Ultrafine-Grained Titanium.” AAPS PharmSciTech 18 6 (2017): 2180–2187.
[3] Fiordaliso, Fabio et al. “Toxicological impact of titanium dioxide nanoparticles and food-grade titanium dioxide (E171) on human and environmental health.” Environmental Science: Nano 4 (2022): 1199–1211.
You may like
Related articles And Qustion
See also
Lastest Price from Titanium dioxide manufacturers

US $1200.00-1100.00/ton2025-08-29
- CAS:
- 13463-67-7
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $100.00-75.00/kg2025-04-21
- CAS:
- 13463-67-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000