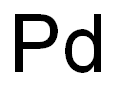The used of Palladium
The leading application of Palladium (Pd) currently is in catalytic converters. Pd is also utilized in jewelry, dentistry, watch-making, blood sugar test strips, aircraft spark plugs, surgical instruments, and electrical contacts. In addition, Pd is used to manufacture professional transverse (concert or classical) flutes. As a commodity, Pd bullion has ISO currency codes of XPD and 964. Pd is one of only four metals with such codes, together with Au, Ag, and Pt. Since it absorbs hydrogen, Pd is a critical component of the controversial cold fusion experiments that began in 1989.

Organic synthesis
When it is finely distributed, as with Pd on carbon, Pd forms a valuable catalyst in, for example, heterogeneous catalytic processes such as hydrogenation, dehydrogenation, and petroleum cracking. Pd is also essential to the Lindlar catalyst, also called Lindlar’s Palladium [A Lindlar catalyst is a heterogeneous catalyst that consists of Pd deposited on CaCO3, which is then poisoned with various forms of Pb or S. It is used for the hydrogenation of alkynes to alkenes (i.e., without further reduction into alkanes).
Many C- C bonding reactions in organic chemistry are aided by Pd compound catalysts. For instance, the Mizoroki-Heck reaction [the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a Pd catalyst (or Pd nanomaterial-based catalyst) to form a substituted alkene.
Suzuki coupling [an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide catalyzed by a Pd(0) complex. It was first published in 1979 by Japanese chemist Akira Suzuki.
Tsuji-Trost reactions [also known as the Trost allylic alkylation or allylic alkylation, is a Pd-catalyzed substitution reaction involving a substrate that contains a leaving group in an allylic position. The Pd catalyst coordinates with the allyl group and then undergoes oxidative addition, forming the π-allyl complex. This allyl complex can then be attacked by a nucleophile, resulting in the substituted product. The scope of this reaction has been expanded to many different carbon, nitrogen, and oxygen-based nucleophiles; many different leaving groups; many different phosphorus, nitrogen, and sulfur-based ligands; and many different metals, although palladium is still preferred.
Electronics
The second largest use of Pd in electronics is in multilayer ceramic capacitors where Pd (and Pd-Au alloy) is utilized for electrodes. Pd (occasionally alloyed with Ni) is employed for component and connector plating in consumer electronics and soldering materials. Hydrogen easily diffuses through heated Pd, and membrane reactors containing Pd membranes are used to produce high-purity H2. Pd is utilized in palladium-hydrogen electrodes in electrochemical research. Pd(II) chloride easily catalyzes carbon monoxide (CO) gas to carbon dioxide (CO2) and is therefore valuable in carbon monoxide detectors.
The hydrogen content in Pd can be related to its magnetic susceptibility, which decreases with the increase of hydrogen and becomes zero for PdH0.62. At higher ratios, the solid solution becomes diamagnetic. Pd is utilized in small quantities (around 0.5%) in some alloys of dental amalgam to reduce corrosion and enhance the metallic luster of the final restoration.
In jewelry
Pd has been utilized as a precious metal in jewelry since 1939 as an alternative to Pt in alloys called “white gold,” where the naturally white color of Pd does not necessitate Rh plating. Pd is much less dense than Pt. Like Au, Pd can be beaten into leaf form as thin as 100 nm. Unlike Pt, Pd may discolor at temperatures above 400℃ and it is relatively brittle. Before 2004, the primary use of Pd in jewelry was the production of white gold. Pd is one of the three most common alloying metals in white gold (Ni and Ag can also be used). Pd-gold is more costly than Ni-gold but rarely triggers allergic reactions (although some cross-allergies with Ni may occur). When Pt became a strategic resource during World War II, many jewelry bands were made from Pd instead. With the casting problem solved, the use of Pd in jewelry increased, initially as the Pt price increased while the price of Pd decreased. Fountain pen nibs made from Au are occasionally plated with Pd when an Ag (rather than Au) look is preferred. Sheaffer has used Pd plating for years, either as an accent on other Au nibs or covering the Au entirely. In the platinotype printing process, photographers make fine art black-and-white prints using Pt or Pd salts. Often used with Pt, Pd provides an alternative to Ag.
You may like
Related articles And Qustion
See also
Lastest Price from Palladium manufacturers

US $2.50-1.00/kg2025-11-03
- CAS:
- 7440-05-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 300tons

US $0.00/kg2025-06-05
- CAS:
- 7440-05-3
- Min. Order:
- 1kg
- Purity:
- 99.95%min
- Supply Ability:
- 1 tons