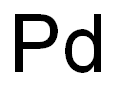The Minerals and Isotopes of Palladium
Introduction

Palladium is a rare and lustrous silvery-white metal. It has 46 electrons arranged in an electronic configuration of [Kr]4d10. Electrons in the s-shell fill the d orbitals because they have less energy. Palladium compounds primarily exist in the 0 and 12 oxidation states, but other less common oxidation states are also known (Table 6.9). Palladium dissolves slowly in concentrated oxidizing acids, nitric acid, and sulfuric acid. When it is finely ground, it can be dissolved in hydrochloric acid. It dissolves readily at room temperature in aqua regia. It also dissolves in fused alkali metal oxides and peroxides.
Minerals
More than 50 minerals are known to contain palladium in their structure. Within the element class, 13 alloys can be found, such as atokite ((Pd, Pt)3Sn), palladium ((Pd, Pt)), and potarite (PdHg). In the sulfide class, 48 minerals are known to contain Pd, for example, arsenopalladinite (Pd8(As, Sb)3), coldwellite (Pd3Ag2S), isomertieite (Pd11Sb2As2), palladoarsenide (Pd2As), and telluropalladinite (Pd9Te4). A single oxide, palladinite (PdO), is known.
Isotopes
Naturally occurring Pd consists of seven isotopes, six of which are stable. The most stable radioisotopes are 107Pd with a half-life of 6.5 million years (found in nature), 103Pd with 16.991 days (synthetic), and 100Pd with 3.63 days (synthetic). Eighteen other radioisotopes have been observed, ranging from 91Pd to 123Pd. These radioisotopes have half-lives of less than 30 minutes, except for 101Pd, which has a half-life of 8.47 hours, 109Pd, which has a half-life of 13.7 hours, and 112Pd, which has a half-life of 21 hours. For isotopes lighter than the most abundant stable isotope, 106Pd, the principal decay mode is electron capture, with the main decay product being Rh. The primary mode of decay for the isotopes heavier than 106Pd is via β decay, with the main product being Ag. Radiogenic 107Ag, as a decay product of 107Pd, was first discovered in 1978 in the Santa Clara meteorite of 1976. The discoverers proposed that the coalescence and differentiation of iron-cored small planets may have happened 10 million years after a nucleosynthetic event. 107Pd versus Ag correlations found in bodies, which have been melted since the accretion of the solar system, must be a reflection of the existence of short-lived nuclides in the early solar system.
You may like
Related articles And Qustion
See also
Lastest Price from Palladium manufacturers

US $2.50-1.00/kg2025-11-03
- CAS:
- 7440-05-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 300tons

US $0.00/kg2025-06-05
- CAS:
- 7440-05-3
- Min. Order:
- 1kg
- Purity:
- 99.95%min
- Supply Ability:
- 1 tons