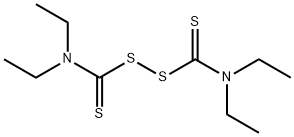
The toxicity of Disulfiram
Introduction
Disulfiram is an organosulfur, an FDA-approved drug used for treating chronic alcoholism under the trade name Antabuse since 1949. Aldehyde dehydrogenase converts acetaldehyde to acetate. Drinking alcohol while taking disulfiram leads to elevated levels of acetaldehyde (product of alcohol metabolism) and precipitation of unpleasant aversive disulfiram–alcohol reaction. Symptoms of this reaction include diaphoresis, flushing, tachycardia, nausea, vomiting, palpitations, hypotension, etc.

Biological activity
Disulfiram exhibits equipotent bactericidal activity against a wide range of Gram-positive pathogens, including E. faecium (16 μg/mL), methicillin-susceptible S. aureus (8 μg/mL), methicillin-resistant S. aureus (MRSA) (8 μg/mL), Staphylococcus epidermidis (1 μg/mL), Mtb (1.56 μg/mL), and nontuberculous mycobacteria (NTM), including Mycobacterium fortuitum and Mycobacterium abscessus (32 μg/mL). It also demonstrated antibacterial activity against a few Gram-negative pathogens, including A. baumannii (16 μg/mL) and K. pneumoniae (64 μg/mL).
Side effects
Because it inhibits aldehyde dehydrogenase and consequently causes the disulfiram-ethanol reaction (vomiting, vertigo, anxiety, cardiovascular effects) after ingestion of alcoholic beverages, however, adverse effects on the central nervous system (psychotic reaction, acute organic brain syndrome, catatonia) may appear as a direct result of the drug itself.
Although it has been suggested that the effectiveness of disulfiram is only as a placebo, there is evidence for the effectiveness being linked to the disulfiram ethanol reaction (DER) due to the pharmacological action and the operant conditioning model. Hepatitis following use of disulfiram has been reported in many studies. There is a close temporal relationship between the occurrence of symptoms and disulfiram intake. Fulminating hepatitis is a rare but potentially fatal adverse reaction that may occur after the use of disulfiram. Disulfiram toxicity may present different clinical aspects: (1) Cytolytic hepatitis with fatal ovulation in 30% of cases (fulminant hepatitis). (2) Severe optic neuritis. (3) Peripheral neuropathy. (4) Encephalitis.
Acute and Short-Term Toxicity
Animal
Disulfiram is not used therapeutically in domestic animals. Its toxicity when ingested in overdose is undefined. The LD50 doses in rats, mice, and rabbits are 500, 1980, and 1800 mg kg−1, respectively.
Human
Acute overdose of disulfiram, in the absence of concomitant ethanol ingestion, may produce hypotension. When taken with ethanol, a constellation of severe reactions, including flushing, vasodilation, pulsating headache, vomiting, and chest pain, may occur. Less commonly, severe reactions, including hypotension with shock, coma, seizures, and myocardial infarction, may occur. An ethanol level as low as 5–10 mg dl−1 may produce this reaction, with fully developed symptoms appearing when ethanol concentrations exceed 50 mg dl−1. These toxic manifestations correlate with increased serum concentrations of acetaldehyde and may persist for 1–2 weeks after cessation of disulfiram use.
References:
[1] PK CHAKRABORTY (RETD) BRIG K D M HARPREET SINGH Maj. CURRENT STATUS OF DISULFIRAM THERAPY[J]. Medical Journal Armed Forces India, 2001, 57 4: 273-350. DOI:10.1016/S0377-1237(01)80013-7.[2] KAPIL BHALLA. Acute Disulfiram Poisoning in a Child: A Case Report and Review of Literature.[J]. Indian Journal of Critical Care Medicine, 2020, 24 3. DOI:10.5005/jp-journals-10071-23371.
You may like
Related articles And Qustion
See also
Lastest Price from Disulfiram manufacturers

US $0.00/g2025-09-11
- CAS:
- 97-77-8
- Min. Order:
- 100g
- Purity:
- 97%
- Supply Ability:
- 20kg/month

US $0.00-0.00/KG2025-04-15
- CAS:
- 97-77-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg