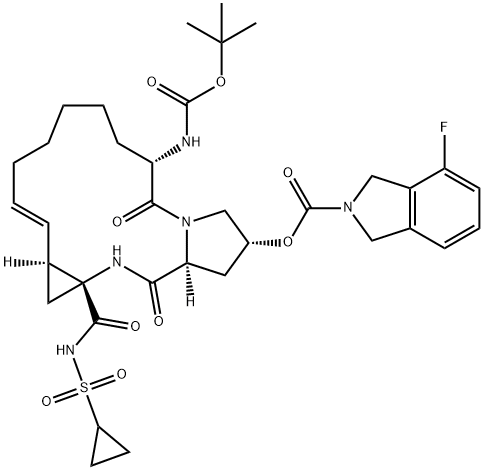
The Synthetic method of Danoprevir
Overview
Danoprevir (Ganovo®) is an orally administered hepatitis C virus (HCV) NS3 protease inhibitor. Recently approved in China for the treatment of newly diagnosed patients with non-cirrhotic genotype 1b chronic hepatitis C. Discovered by InterMune Inc. and Array Biopharma Inc., the rights to license danoprevir were acquired by Roche in 2006, who later partnered with Aslectis for the co-development and commercialization of the drug in China[1].
Mode of action
Danoprevir inhibits the NS3 protease active site through a two-step binding mechanism involving an initial collision complex rapidly isomerizing to a highly stable complex displaying unusually slow dissociation. With a calculated complex half-life of roughly 5 h, the observed binding kinetics distinguish danoprevir from other related macrocyclic inhibitors of NS3. Danoprevir is also a substrate of cytochrome P4503A. To maximize its effectiveness, danoprevir is coadministered with the CYP3A inhibitor/inducer ritonavir, as well as the antiviral drugs peginterferon alfa-2a and ribavirin, both of which are also currently used to treat hepatitis C.
The drug target of danoprevir is HCV NS3, which consists of a zinc-dependent serine protease at the N terminus (amino acids: 1 to 180) and an ATP-dependent RNA helicase at the C terminus (amino acids: 181 to 631). During the viral maturation, HCV NS3 serine protease is an indispensable enzyme that cleaves HCV precursor polyproteins at four junctions (NS3-NS4A, NS4A-NS4B, NS4B-NS5A, NS5A-NS5B). This catalytic processing releases five non-structural viral proteins (NS3, NS4A, NS5B, NS5A, NS5B) for their subsequent maturation. The activation of NS3 protease indispensably requires binding its cofactor NS4A, a single-pass transmembrane protein that locates and stabilizes NS3 protease facing towards the cytosol. NS3 helicase coupled with adenosine triphosphate (ATP) is responsible for the RNA translocation and unwinding[2].
Synthetic method
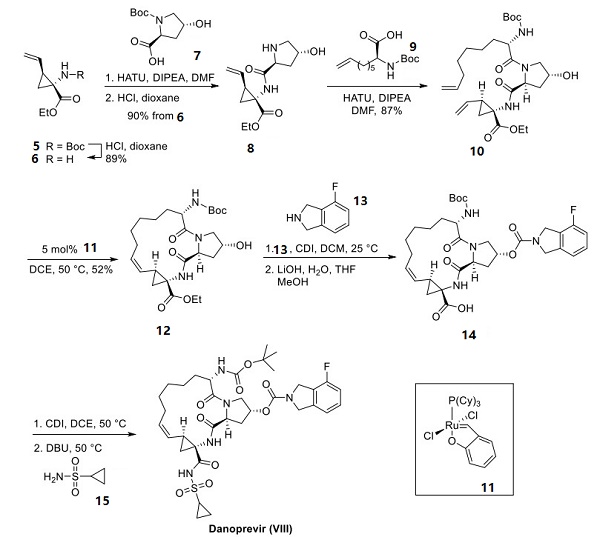
Scientists at Boehringer-Ingelheim have reported a concise approach to the building block cyclopropyl aminoester 5 (a substructural component of several macrocyclic antiviral drugs) on the multikilogram scale, and this approach is described below. The hydrochloride salt of ethyl glycine 1 was condensed with benzaldehyde to form imine 2, making way for a cyclative dialkylation with 2-butene-1,4-dibromide 3 through a sequential SN2−SN2′ reaction. Acidic hydrolysis of the imine and N-Boc protection yielded racemic vinylcylopropane 4 in 65−70% yield over three steps[3]. Enzymatic resolution using Alcalase 2.4L allowed for selective saponification of the undesired (1S,2R)-carboxylate, providing the desired (1R,2S) enantiomer 5 in quantitative conversion (including mass recovery of the acid).
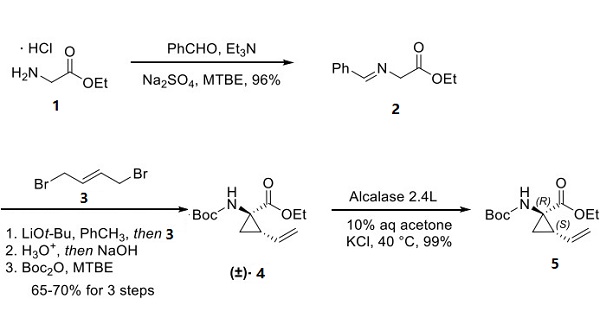
After that, deprotection of the amine within 87 to give 88 and amide formation mediated by hexafluorophosphate azabenzotriazole tetramethyluronium (HATU) with carboxylic acid 89 (shown below). This was followed by a second Boc-deprotection step to afford amino alcohol 90 in 80% yield over the three-step sequence. HATU-mediated amide coupling with commercial carboxylic acid 91 provided alkene 92, which, when treated with Hoveyda first-generation metathesis catalyst 93, afforded macrocycle 94 in 52% yield. Carbonyldiimidazole (CDI)-mediated coupling of 94 and isoindoline 95 followed by basic hydrolysis yielded carboxylic acid 96. This acid was then reacted with CDI and cyclopropylsulfonamide 97, furnishing danoprevir.
References
[1] Anthony Markham, Susan J Keam. “Danoprevir: First Global Approval.” Drugs 78 12 (2018): 1271–1276.
[2] Miao Miao. “Danoprevir for the Treatment of Hepatitis C Virus Infection: Design, Development, and Place in Therapy.” Drug Design, Development and Therapy (2020): 2759–2774.
[3] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
You may like
See also
Lastest Price from Danoprevir manufacturers

US $15.00-10.00/KG2021-07-13
- CAS:
- 850876-88-9
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton