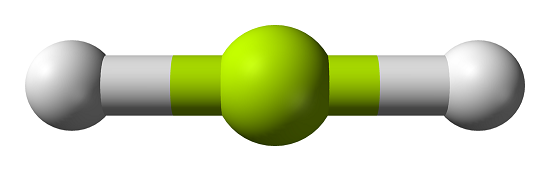
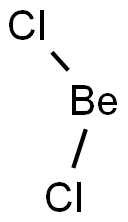The Structure of Beryllium Chloride
Beryllium chloride (BeCl2) is available as colorless to yellow crystals. It decomposes rapidly on contact with water producing hydrogen chloride, and attacks many metals in the presence of water. Beryllium chloride emits irritating or toxic fumes (or gases) in a fire.
Beryllium chloride is used as a catalyst to accelerate many organic reactions, and beryllium chloride is the electrolyte used along with NaCl in the electrolytic process to produce beryllium metal.

The Polarity of BeCl2
The molecule’s polarity depends upon certain parameters of the molecule such as the shape of the molecule, net dipole moment, and the distribution of charges in the molecule.
Chlorine (E.N = 3.16) is more electronegative than beryllium (E.N = 1.57). An electronegativity difference of 1.59 units is present between these two bonded atoms. Due to this electronegativity difference, chlorine strongly attracts the shared electron cloud from the Be-Cl bond in the BeCl2 molecule. The bonded electrons are held significantly close to the chlorine.
The Cl-atom thus gains a partial negative (Clδ-) charge, while the beryllium atom, less electronegative, obtains a partial positive (Beδ+) charge. In this way, oppositely charged poles develop in the BeCl2 molecule. Consequently, each Be-Cl bond in the BeCl2 molecule is polar. This is called the bond polarity of BeCl2.
However, Beryllium Dichloride is a nonpolar molecule as there is zero net dipole moment. The molecule has linear geometry hence the dipole moments in the molecule get nullified.
The Electron Structure of BeCl2[1]
The simple compound, beryllium chloride, offers an opportunity to study halogen bridge bonding. Beryllium chloride is isomorphous with dimethylberyllium and silicon disulfide. The orthorhombic unit, a0=9.86, b0=5.36, c0=5.26A contains four beryllium chloride molecules. The structure consists of continuous chainswith an essentially tetrahedral configuration about beryllium. The Cl–Be–Cl bond angle within the four membered rings is 98.2° which is less than the tetrahedral angle, as is found in SiS2, instead of more, as in dimethylberyllium. The bond angles indicate that all bonds in beryllium chloride contain an electron pair, so that chlorine uses an unshared pair in forming bridge bonds. Hence, chlorides isomorphous with electron deficient metal alkyls are not to be classified as electron deficient compounds.
Reference
[1] R. E. Rundle; Paul H. Lewis. Electron Deficient Compounds. VI. The Structure of Beryllium Chloride. J. Chem. Phys. 20, 132–134 (1952).