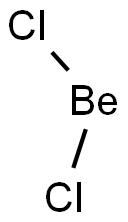Is BeCl2 a polar or a nonpolar compound?
Dec 25,2023
Beryllium chloride (BeCl2) is a non-polar molecule. The electronegativity difference between beryllium (1.57) and chlorine (3.16) is 1.59 making putting it within the polar covalent range.
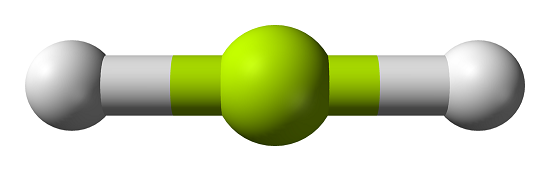
BeCl2 (Beryllium chloride) is non-polar due to its symmetrical (linear-shaped) geometry. Each Be-Cl bond in the BeCl2 molecule is polar due to an electronegativity difference between the bonded Be and Cl atoms.
The electronegativity of the chlorine (Cl) atom is greater than the beryllium (Be) atom. The Cl atom strongly attracts the shared electron pair in the Be-Cl bond.
Thus, BeCl2 is a nonpolar molecule, the linearly arranged chlorine's balance out around the central beryllium atom.
You may like
The Applications of Sodium tungstate dihydrate
Oct 10, 2025