The synthesis method of Polatuzumab vedotin
Overview
Polatuzumab vedotin (polatuzumab vedotin-piiq) is an antibody-drug conjugate comprising a monoclonal antibody against CD79b (a B cell receptor component) covalently conjugated to the anti-mitotic cytotoxic agent monomethyl auristatin (MMAE) via a cleavable linker. After binding to CD79b on the B-cell surface, polatuzumab vedotin is internalized, and the linker is cleaved, releasing MMAE into the cell, inhibiting division and inducing apoptosis. The antibody–drug conjugate polatuzumab vedotin has been approved to treat relapsed or refractory diffuse large B-cell lymphoma. The drug recognizes the CD79b protein on B cells and kills them with a molecule that prevents tubulin polymerization[1–2].
Synthesis method
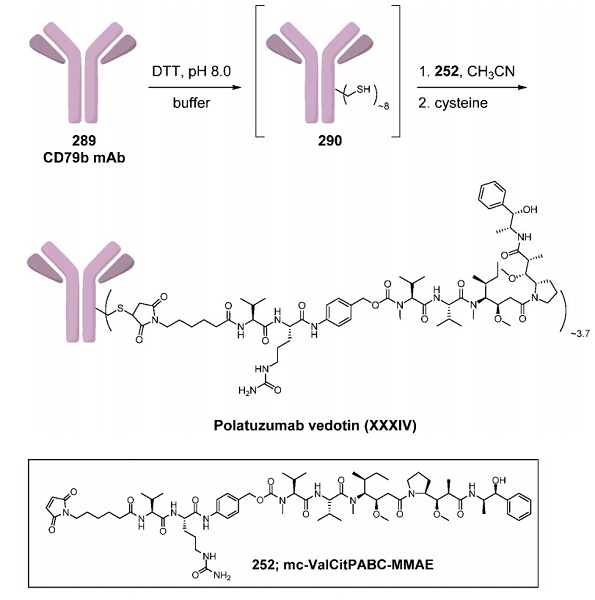
Although an explicit preparation of polatuzumab vedotin was not found in the primary or patent literature, the drug was prepared using Seagen technology. Thus, the synthesis is described above and is very similar to that described for brentuximab vedotin. Anti-CD79b antibody 289 was dissolved in PBS, and 50 mM borate was added. After the pH was adjusted to 8, dithiothreitol (DTT) was added to reduce the intrachain disulfide bonds to reveal approximately eight free sulfhydryl groups (290). The reaction of these free thiol groups with 4 equiv of linker−payload mc-ValCitPABC-MMAE (252) in acetonitrile followed by reoxidation of the unreacted sulfhydryl groups and quenching of excess vcMMAE with cysteine provided polatuzumab vedotin with a DAR of approximately 3.7.
References
[1] Deeks, Emma D. “Polatuzumab Vedotin: First Global Approval.” Drugs 79 13 (2019): 1467–1475.
[2] “Polatuzumab Vedotin Approved for DLBCL.” Cancer discovery (2019): OF2.
[3] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.


