Evaluation of the efficacy safety and toxicity of Amphotericin B
What is Amphotericin B?
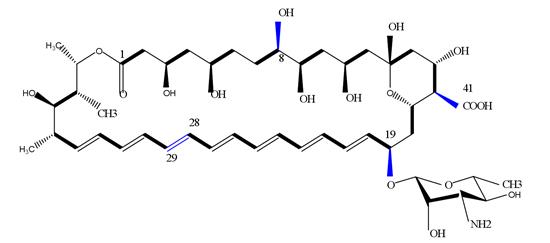
Amphotericin B (AmB) is the oldest and for decades the only available agent for the treatment of life-threatening invasive fungal diseases, known for its broad-spectrum fungicidal activity against a wide range of yeasts and moulds. However, amphotericin B injection can cause serious side effects. it should only be used to treat potentially life-threatening fungal infections and not to treat less serious fungal infections of the mouth, throat, or vagina in patients with a normal immune system (body's natural protection against infection). the major drawbacks of current formulations remain their toxicity, limited availability for intravenous use, and the higher costs associated with better-tolerated lipid formulations.

Mechanism of Action
Amphotericin B acts by binding to ergosterol in the cell membrane of most fungi. After binding with ergosterol, it causes the formation of ion channels leading to loss of protons and monovalent cations, which results in depolarization and concentration-dependent cell killing. Additionally, amphotericin B also produces oxidative damage to the cells with the formation of free radicals and subsequently increased membrane permeability. Additionally, amphotericin B has a stimulatory effect on phagocytic cells, which assists in fungal infection clearance.
Side Effects AND Toxicity
Side effects such as infusion-related or nephrotoxicity occur in about 80% of patients taking amphotericin B. Its most common side effects include loss of potassium and magnesium, allergic reactions, fever, and nephrotoxicity. Other potentially uncommon side effects include demyelinating encephalopathy in patients with systemic irradiated bone marrow transplantation or those being treated with cyclosporine. Long-term administration of erythropoietin has been associated with normochromic, normocytic anaemia due to low erythropoietin concentrations. The primary cause of this factor is due to the interaction of amphotericin B with cholesterol in human cell membranes.
Amphotericin exhibits infusion-related toxicity, which accounts for its extended administration times. Infuse slowly over 3 hours; rapid infusion can cause cardiotoxicity. Due to the similarity of mammalian and fungal membranes, which both contain sterols (the therapeutic target for amphotericin B), amphotericin B can exhibit cellular toxicity.
Related Preparations
In order to improve the therapeutic index of amphotericin B, three lipid-associated formulations were developed, including amphotericin B lipid complex (ABLC), liposomal amphotericin B (L-AmB), and amphotericin B colloidal dispersion (ABCD). ABLC is the largest lipid agent. Because of its size, it is rapidly taken up by macrophages and is isolated to tissues with mononuclear phagocytic systems (e.g. liver and spleen). As a result, it has a lower circulating amphotericin B serum concentration than conventional preparations, which is reflected in a significant increase in volume of distribution and clearance. Lung levels are much higher than those achieved with other lipid-related preparations.The recommended therapeutic dose of ABLC is 5 mg/kg/day. Due to its small size and negative charge, L-AmB avoids substantial recognition and uptake by the mononuclear phagocyte system. As a result, a single dose of L-AmB results in a much higher peak plasma level (Cmax) than conventional amphotericin B deoxycholate and a much larger area under the concentration-time curve. Patients treated with L-AmB tend to have the highest tissue concentrations in the liver and spleen and much lower tissue concentrations in the kidney and lung. The recommended therapeutic dose is 3-6 mg/kg/day.
Following intravenous infusion, the ABCD complex remains largely intact and is rapidly cleared from the circulation by cells of the macrophage phagocytic system. On a mg to mg basis, the Cmax achieved is lower than that achieved with conventional amphotericin B, although larger doses of ABCD given produce absolute levels similar to those of amphotericin B. Therefore, the dose administered should not exceed 3-4 mg / kg / day. Despite the significant safety benefits of lipid products, the few comparative clinical trials of lipid-related agents that have been completed have not demonstrated clinically important differences in efficacy between these drugs and amphotericin B. The few comparative clinical trials that have been completed have not demonstrated clinically important differences in efficacy between these agents and amphotericin B.
In the only published study, L-AmB had significantly better clinical success and mortality rates compared with amphotericin B deoxycholate; there were no differences in microbiological outcomes between treatment groups. Lipid-related agents were not significantly superior to amphotericin B deoxycholate for the treatment of AIDS-related acute cryptococcal meningitis with respect to the clinical or microbiological outcomes studied. In all trials that specifically examined nephrotoxicity, the lipid-associated formulations were significantly less nephrotoxic than amphotericin B deoxycholate. Infusion-related reactions occurred less frequently with L-AmB than with amphotericin B deoxycholate; however, ABCD had the same or more frequent infusion-related reactions than conventional amphotericin B, which led to the discontinuation of at least one clinical trial. This particular lipid formulation is no longer commercially available. For the treatment of most invasive fungal infections, lipid formulations of amphotericin B provide a safer alternative to traditional amphotericin B with at least equivalent efficacy.
References:
[1] MARIA AIGNER; Cornelia L F. Encochleated Amphotericin B: Is the Oral Availability of Amphotericin B Finally Reached?[J]. Journal of Fungi, 2020. DOI:10.3390/jof6020066.
[2] HAMILL R J. Amphotericin B formulations: a comparative review of efficacy and toxicity.[J]. Drugs, 2013, 73 9: 919-934. DOI:10.1007/s40265-013-0069-4.
You may like
Related articles And Qustion
See also
Lastest Price from Amphotericin B manufacturers
US $0.00/kg2025-11-21
- CAS:
- 1397-89-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $1.00-4.00/KG2025-09-11
- CAS:
- 1397-89-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG