The synthesis method of neopentyl glycol
Introduction
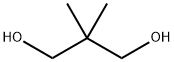
Neopentyl glycol (IUPAC name: 2,2-dimethylpropane-1,3-diol) is an organic chemical compound. It is a white crystalline solid with a melting point of 130-131℃. It is used to synthesise polyesters, paints, lubricants, and plasticizers. When used in the manufacture of polyesters, it enhances the stability of the product towards heat, light, and water.
Uses
The unabated growth in demand for high-quality polymeric materials used in various industries resulted in a growing interest in neopentyl glycol (NPG). NPG is widely used to prepare saturated and unsaturated polyester resins, polyethers, polyurethanes, and high-quality alkyd resins. NPG is also used to produce synthetic lubricants, drugs, pesticides, plasticizers, paints, varnishes, and dyes. Introducing a neopentyl structure to the NPG-derived products enhances their stability towards hydrolysis, high temperature, and light exposure. Therefore, NPG finds application, in particular, in synthesising high-quality coatings (mainly solvent-free powder coatings) that are used, e.g., in the automotive, construction, and shipbuilding industries. Moreover, NPG shows properties characteristic of phase-changing materials (PCMs) (solid-solid phase transition at 43 °C). PCMs are used in many fields due to their ability to accumulate and release energy in the temperature range of the phase transition.
Synthesis
Neopentyl glycol can be obtained by the crossed aldol condensation of isobutyraldehyde with formaldehyde or paraformaldehyde to produce hydroxypivaldehyde as an intermediate. Then, HPA can be converted to NPG either by a Cannizzaro reaction with formaldehyde (variant 1) or a hydrogenation reaction (variant 2). These two methods are of industrial importance. Other methods of NPG synthesis are: dimethylmalonic acid or esters reduction with hydrogen or lithium aluminum hydride, 2-methyl-1,2-epoxypropane hydroformylation, and the subsequent reduction.
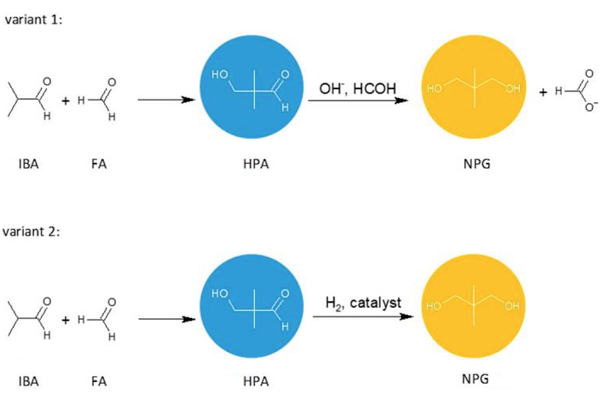
In variant 1, the condensation and Cannizzaro reactions can be carried out in one or two separate stages. The Cannizzaro reaction, performed in the presence of a concentrated inorganic hydroxide solution, gives, apart from NPG, an equimolar amount of formic acid, which is neutralized by the alkaline catalyst. This salt has no useful application, making the method ineffective and industrially unattractive. Recently, however, an interesting method for carrying out polyol synthesis via the Cannizzaro reaction was disclosed in Oxea's patent. In this method, two different polyols are obtained simultaneously in the reaction of two aliphatic aldehydes with formaldehyde in the presence of aqueous solutions of KOH, NaOH or Ca(OH)2. The process takes place in two stages. In the first stage, FA reacts with the first aldehyde, using a 3- to 12-fold excess of formaldehyde relative to the aldehyde, and the reaction is carried out at a temperature ranging from 20 to 65 °C until at least 50% conversion of aldehyde is achieved. Then, without any work-up and isolation, the reaction mixture is reacted with the second aldehyde at a temperature ranging from 30 to 75 °C, with a molar ratio of FA to aldehyde being 2:1 to 7:1. The process is carried out until the aldehydes are fully converted. According to the authors, the simultaneous, consecutive synthesis allows the attainment of polyols with high yields and selectivity.
In variant 2, the first step—condensation of isobutyraldehyde with formaldehyde is commonly conducted in the presence of a basic catalyst such as trialkylamines, sodium or potassium carbonates, anion exchangers or layered double hydroxides. The selectivity of HPA synthesis using tertiary amines as catalysts is usually higher compared to the processes carried out with inorganic bases. However, as with inorganic bases, tertiary amines can form salts by reacting with organic acids. These acids are mainly formed in the Cannizzaro reaction or are introduced with starting materials, e.g., formic acid from commercial formaldehyde, which is usually used as an aqueous solution containing methanol. The resulting salts cannot be easily separated from the hydroxy aldehyde and are usually directed with hydroxy aldehyde to the hydrogenation reactor. Their presence in the HPA stream may lead to the deactivation of the metal catalyst used in the hydrogenation reaction. Moreover, they may promote the decomposition of the aldol condensation product during the distillation of the reaction mixture at high temperatures, thereby reducing the yield of the desired product. Amine salts can also adversely affect the color and/or odor of HPA-derived products.
References:
[1] ANMING YANG. Facile and Rapid Synthesis of Neopentyl Glycol in a Continuous-Flow Microreactor[J]. Chemical Engineering & Technology, 2021, 45 1: 1-204. DOI:10.1002/ceat.202100164.[2] EDYTA MONASTERSKA. Development of Methods for the Synthesis of Neopentyl Glycol by Hydrogenation of Hydroxypivaldehyde.[J]. ACS Applied Energy Materials, 2021. DOI:10.3390/molecules26195822.
You may like
Related articles And Qustion
Lastest Price from neopentyl glycol manufacturers

US $10.00/kg2025-04-21
- CAS:
- 126-30-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 126-30-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20MT