Neopentyl Glycol: A Versatile Diol for Advanced Polymer Applications and Radiopharmaceutical Development
General Description
Neopentyl glycol is a versatile diol widely used in the production of high-quality polymeric materials, including polyester resins, polyethers, and polyurethanes. Its structural properties confer exceptional stability, making it ideal for applications in durable coatings across various industries. Additionally, neopentyl glycol serves as a crucial scaffold in radiopharmaceutical development, particularly for creating radiohalogenated compounds used in radiotheranostics. Its resistance to metabolic degradation and favorable biodistribution profiles enhance its effectiveness in targeted therapies. These qualities ensure that neopentyl glycol remains a key component in both material science and advanced medical applications, supporting ongoing research and development efforts.

Figure 1. Neopentyl glycol
Overview
Properties
Neopentyl glycol is a versatile diol compound renowned for its application in creating a wide range of high-quality polymeric materials. The demand for neopentyl glycol has surged due to its significant role in formulating saturated and unsaturated polyester resins, polyethers, and polyurethane compounds. The unique structure of neopentyl glycol enhances the stability of the materials produced, making them resistant to hydrolysis, high temperatures, and light exposure. This characteristic is particularly beneficial in industries that require durable coatings, such as automotive, construction, and shipbuilding. Additionally, neopentyl glycol is instrumental in the production of synthetic lubricants, pharmaceuticals, pesticides, plasticizers, paints, varnishes, and dyes, highlighting its broad applicability across various sectors.1
Synthesis Methods
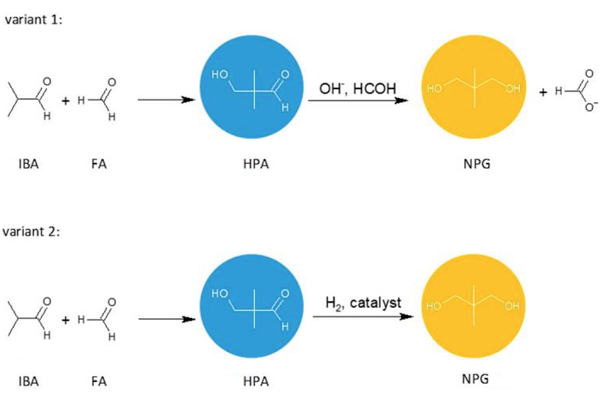
Neopentyl glycol can be obtained by the crossed aldol condensation of isobutyraldehyde with formaldehyde or paraformaldehyde to produce hydroxypivaldehyde as an intermediate. Then, HPA can be converted to NPG either by a Cannizzaro reaction with formaldehyde or by a hydrogenation reaction. These two methods are of industrial importance. Other methods of NPG synthesis are: dimethylmalonic acid or esters reduction with hydrogen or lithium aluminum hydride, 2-methyl-1,2-epoxypropane hydroformylation, and the subsequent reduction.1
Applications in Radiopharmaceutical Development
Neopentyl Glycol in Radiotheranostics
Neopentyl glycol has emerged as a vital scaffold for developing radiotheranostic systems due to its exceptional stability and performance in vivo. Recent studies have shown that the derivatization of neopentyl glycol allows for the preparation of radiohalogenated compounds, specifically those involving radioiodine and astatine. The versatility of neopentyl glycol enables the creation of analogues with varying hydroxyl groups that retain stability against nucleophilic substitutions. This quality is crucial for the synthesis and application of radiopharmaceuticals intended for both therapeutic and diagnostic purposes. As a radiolabeling scaffold, neopentyl glycol facilitates the incorporation of radioisotopes while ensuring the integrity of the compound during biological interactions. 2
Stability and Metabolism
In the context of in vivo applications, neopentyl glycol demonstrates remarkable resistance to metabolic degradation. The 125I-labeled compounds synthesized with neopentyl glycol were notably stable against cytochrome P450-mediated metabolism, which is a common pathway for chemical modification in biological systems. Of particular interest, the 211At-labeled version of neopentyl glycol exhibited similar stability profiles, resisting both nucleophilic substitution and metabolic alterations. These findings underscore the unique ability of neopentyl glycol to maintain structural integrity under physiological conditions, making it an ideal scaffold for delivering radiohalogenated compounds that can be used in targeted therapy. 2
Biodistribution and Excretion
Neopentyl derivatives were evaluated as a scaffold to prepare radioiodinated and astatinated probes of high in vivo stability. All the tested radiolabeled neopentyl derivatives remained stable against nucleophilic attack. However, only a neopentyl derivative with two hydroxyl groups (neopentyl glycol) provided 125I- and 211At-labeled compounds highly stable against CYP-mediated metabolism. Both compounds registered biodistribution profiles similar in mice with in vivo dehalogenation hardly observed. Most of the radioactivity was excreted in the urine as GCs for both compounds. These findings indicate that neopentyl glycol would constitute a useful scaffold for developing a radiotheranostic system with radioiodine and astatine as the radiolabels for further applications.2
References:
[1] EDYTA MONASTERSKA. Development of Methods for the Synthesis of Neopentyl Glycol by Hydrogenation of Hydroxypivaldehyde.[J]. ACS Applied Energy Materials, 2021. DOI:10.3390/molecules26195822.[2] HIROYUKI SUZUKI*. Neopentyl Glycol as a Scaffold to Provide Radiohalogenated Theranostic Pairs of High In Vivo Stability[J]. Journal of Medicinal Chemistry, 2021, 64 21: 15515-16318. DOI:10.1021/acs.jmedchem.1c01147.
You may like
Related articles And Qustion
Lastest Price from neopentyl glycol manufacturers

US $10.00/kg2025-04-21
- CAS:
- 126-30-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 126-30-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20MT