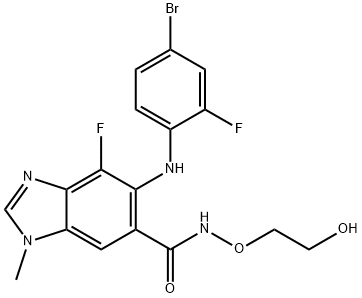
The synthesis method of Binimetinib
Description
Binimetinib is a non-ATP competitive mitogen-activated protein kinase 1/2 (MEK1/2) inhibitor discovered by Array BioPharma and approved for use in combination with the BRAF inhibitor encorafenib (Braftovi) for the treatment of BRAF V600E/K mutant melanomas.
While the use of BRAF inhibitors alone provides efficacy in BRAF mutant malignancies, including 40−50% of metastatic melanomas, re-emergence of disease is common. A mechanistic understanding of this finding has emerged: in patients treated with BRAF inhibitors, simultaneous inhibition of wild-type BRAF paradoxically leads to activation of the mitogen-activated protein kinase (MAPK) pathway, resulting in resistance and the development of keratoacanthomas and squamous cell carcinomas. Supplementing Braftovi therapy with binimetinib blocks MAPK-induced MEK1/2 signaling, resulting in increased progression-free survival (PFS) and overall survival (OS) compared to encorafenib or vemurafenib (another BRAF inhibitor). Binimetinib is currently marketed in the U.S. by Pfizer after it acquired Array BioPharma in 2019.
Synthetic method
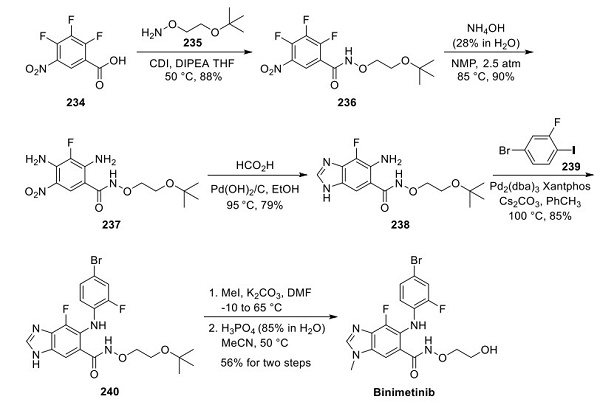
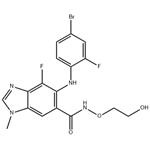
The most recent and most extensive demonstrated scale (0.1 mol) route to binimetinib avoids silica gel chromatography and is described above[1]. A noteworthy aspect of this sequence is the effective introduction and characterization of multiple reactive nitrogen atoms in building the pentasubstituted benzoyl core. Coupling 2,3,4-trifluoro-5- nitrobenzoic acid with O-alkyl hydroxylamine 235 using CDI and diisopropylethylamine afforded alkoxy amide 236 in high yield. Ammonium hydroxide displacement of the activated fluorine atoms within compound 236 required pressurized conditions employing a steel bomb to furnish 237. Benzimidazole formation was then realized by subjecting nitroaniline 237 to formic acid and palladium hydroxide on carbon, providing 238 after recrystallization from EtOAc and heptane. An impressive palladium-catalyzed arylation of 238 with 1-iodo-4-bromo-2-fluorobenzene (239) exclusively provided the hindered primary aniline in the presence of the unsubstituted benzimidazole and amide N-H functionalities, giving 240 in good conversion following recrystallization from EtOAc and heptane. A second chemoselective functionalization, methylation of the benzimidazole N-1 position, was cleanly affected using methyl iodide and K2CO3 in DMF. Recrystallization from MeOH yielded the penultimate intermediate directly treated with excess aqueous phosphoric acid in acetonitrile for tert-butyl ether cleavage. Recrystallization gave binimetinib a 56% yield over the two steps.
References
[1] ANDREW C. FLICK. Synthetic Approaches to New Drugs Approved during 2018[J]. Journal of Medicinal Chemistry, 2020, 63 19: 10652-10704. DOI:10.1021/acs.jmedchem.0c00345.
You may like
Related articles And Qustion
Lastest Price from Binimetinib manufacturers
US $0.00-0.00/kg2025-08-22
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00/g2025-01-13
- CAS:
- 606143-89-9
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month