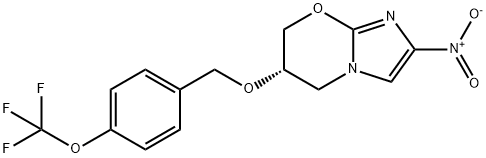
The Synthesis method and Pharmacokinetics of Pretomanid (PA 824)
Description
Pretomanid (also known as PA 824) is a nitroimidazooxazine antimycobacterial drug with a complex mechanism of action that involves inhibition of cell wall mycolic acid biosynthesis under replicating (aerobic) conditions and respiratory poisoning through the generation of reactive nitrogen species, including nitric oxide (NO), under non-replicating (anaerobic) conditions[1].
Synthesis method
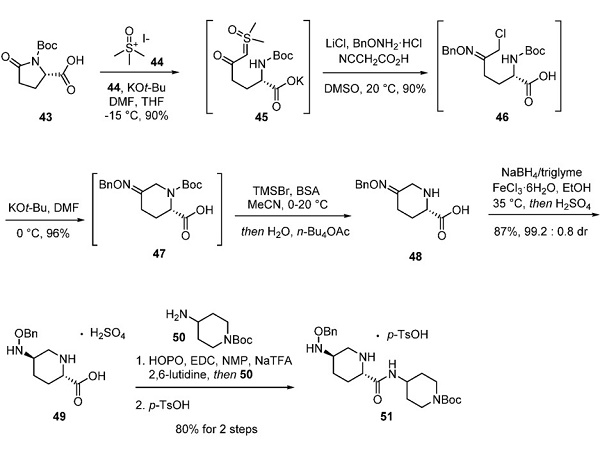
The synthesis of pretomanid has attracted considerable attention within the synthetic community. Early patents discussing the preparation of pretomanid incorporated 2,4-dinitro-1H-imidazole as the starting material, which exhibits explosive characteristics detrimental to safe, large-scale procedures. Although several methods to prepare the drug have been published in several patents, a route to pretomanid that avoids the use of dinitroimidazole is shown below[2].

Chloroimidazole 1 was reacted with silyl-protected epoxide 2 under primary conditions. Following crystallization from cyclohexane, alcohol 3 was isolated in 67% yield over two steps. Exposure of alcohol 3 to benzyl bromide 4 in DMF under cryogenic conditions formed benzyl ether 5 in 61% yield. Silyl deprotection with TBAF liberated the primary alcohol, which underwent cyclization upon treatment with methanolic potassium hydroxide. Recrystallization from isopropanol and cyclohexane afforded pretomanid a 57% yield over three steps.
Pharmacokinetics
Pretomanid pharmacokinetics after oral, single-dose administration were approximately dose-proportional over a dose range of 50–200 mg and less than dose-proportional over a dose range>200–1000 mg[3]. After a single 200 mg dose of pretomanid was administered with food to healthy adults, the mean Cmax of 2.0 μg/mL was achieved in a median of 5.0 h, and the mean AUC∞ was 53.0 μg h/mL. Pretomanid is≈86.4% bound to plasma protein, and the estimated mean apparent volume of distribution is 97 L. A Steady state was achieved≈4–6 days after repeated pretomanid 200 mg once daily under fasted conditions in the same population. The accumulation ratio was≈2 under fasted conditions; the steady state mean Cmax (1.7 μg/mL) was achieved in a median of 4.5 h, and the mean AUC24 was 30.2 μg h/mL.
References
[1] Jeffrey L Ambroso. “Assessment of the Carcinogenic Potential of Pretomanid in Transgenic Tg.rasH2 Mice.” International Journal of Toxicology (2022): 367–379.
[2] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
[3] Keam, S. “Pretomanid: First Approval.” Drugs 79 1 (2019): 1797–1803.
See also
Lastest Price from Pretomanid manufacturers
US $0.00/kg2025-11-21
- CAS:
- 187235-37-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00-0.00/mg2025-05-21
- CAS:
- 187235-37-6
- Min. Order:
- 10mg
- Purity:
- 99%+ HPLC
- Supply Ability:
- 1000