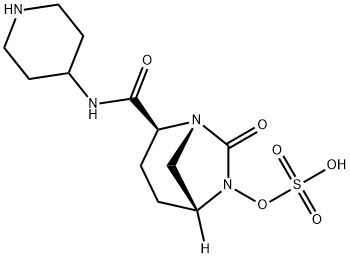
How to synthesize Relebactam?
Overview
Relebactam, formerly MK-7655, is a DBO that promises to contribute to the renaissance in antimicrobial chemotherapy[1]. When combined with imipenem, relebactam is effective against Enterobacteriaceae with known KPC carbapenemases, AmpCs, and/or extended-spectrum β-lactamases (ESBLs)[2].
Specific steps of synthesis
The preparation of Relebactam can be mainly divided into the following two parts:
Preparation of Relebactam Piperidine 51
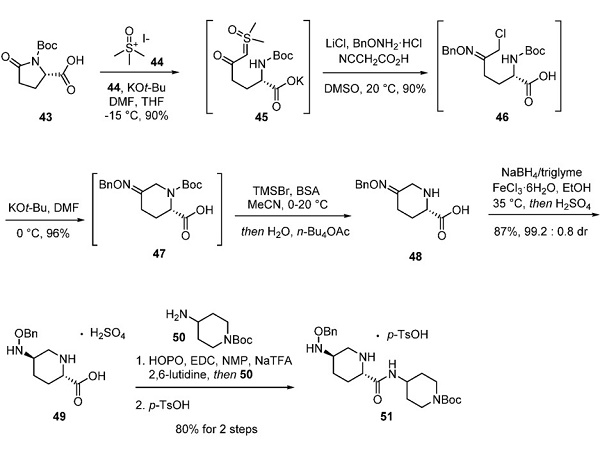
(S)-N-Bocpyroglutamic acid (43) underwent a ring-opening reaction upon treatment with trimethylsulfoxonium iodide (44) and potassium tert-butoxide, resulting in intermediate 45, which could be carried forward without workup. Condensation of 45 with (benzyloxy)ammonium chloride in the presence of lithium chloride and cyanoacetic acid resulted in α- chlorooxime 46. Critical to the reaction’s efficiency, DMS suppressed unintended chloride displacement within 46 by residual iodide from the previous ylide reaction—subjection of 46 to potassium tert-butoxide in chilled DMF-secured piperidine 47. Trimethylsilyl bromide and N, O-bis- (trimethylsilyl)acetamide (BSA) removed the Boc group to furnish piperidine 48, which existed as a rapidly equilibrating mixture of (E)- and (Z)-oximes. Studies were conducted to optimize the conditions for the reduction of oxime 48. Ultimately, a mixture of sodium borohydride and triglyme delivered the desired product, which was further resolved by recrystallization from 0.05 M sulfuric acid to allow for isolation of hydroxylamine 49 as the H2SO4 salt in excellent yield and diastereomeric purity. Salt metathesis was required to convert the poorly soluble salt 49 to its trifluoroacetate counterpart prior to amidation with nucleophile 50. Subsequent tosylate salt formation provided piperidine 51.
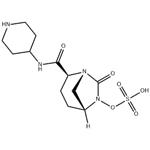
Synthesis of Relebactam
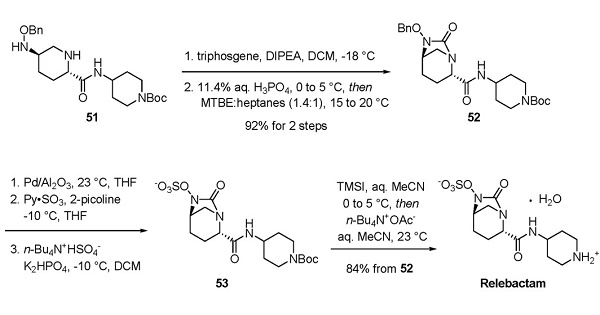
Exposure of 51 to triphosgene followed by acidic workup and ethereal recrystallization resulted in an intramolecular cyclization to secure bridged urea 52. Reduction, sulfonylation, and buffering conditions then facilitated arrival at 53, which underwent careful fit-for-purpose Boc removal using conditions involving trimethylsilyl iodide to afford relebactam as a zwitterionic monohydrate[3].
References
[1] Krisztina M Papp-Wallace. “Relebactam Is a Potent Inhibitor of the KPC-2 β-Lactamase and Restores Imipenem Susceptibility in KPC-Producing Enterobacteriaceae.” Antimicrobial Agents and Chemotherapy 62 6 (2018).
[2] Timothy A. Blizzard . “Discovery of MK-7655, a β-lactamase inhibitor for combination with Primaxin®.” Bioorganic Medicinal Chemistry Letters 24 3 (2014): Pages 780-785.
[3] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
See also
Lastest Price from Relebactam manufacturers
US $0.00/kg2025-11-21
- CAS:
- 1174018-99-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise
US $8.00-1.00/KG2024-03-29
- CAS:
- 1174018-99-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available