The structure of Mg2Sn
Mg2Sn is a promising mid-temperature thermoelectric (TE) material with earth-abundant, low-cost, and non-toxic elements. Currently, the TE performance of p-type Mg2Sn is still poor due to a lower power factor (PF) and a higher lattice thermal conductivity (κlat) than those of n-type Mg2Sn[1].
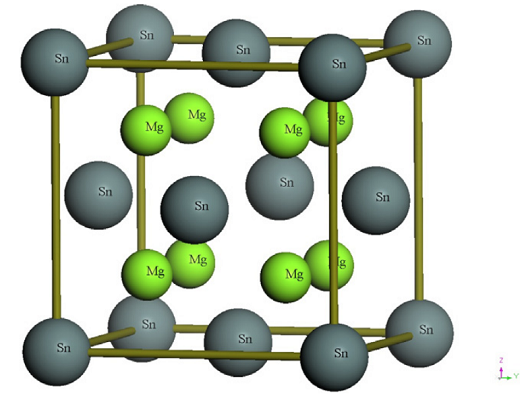
Mg₂Sn is Fluorite structured and crystallizes in the cubic Fm̅3m space group. Mg is bonded to four equivalent Sn atoms to form a corner and edge-sharing MgSn₄ tetrahedra mixture. All Mg-Sn bond lengths are 2.93 Å. Sn is bonded in a body-centered cubic geometry to eight equivalent Mg atoms.
The hexagonal phase of Mg2Sn was reported as the equilibrium structure in the Mg–Sn system at high pressures and high temperatures. This phase was described by Cannon and Conlin as a new dense form of Mg2Sn and later confirmed by Dyuzheva et al. These last authors studied the temperature-pressure rela-tionship of the phase transformation,and reported acombination of quenching and pressure release procedurethat allows the retention of the metastable phase up tonormal conditions. They calculated α=13.18 A and c=6.99 A for the high-pressure phase of hexagonal Mg2Sn. Later on, they described the structure of the metastablephase with a trigonal unit cell,with α=13.19 A and c=13.28 A[2].
References
[1] G. Urretavizcaya , G.O. Meyer. “Metastable hexagonal Mg2Sn obtained by mechanical alloying.” Journal of Alloys and Compounds 339 1 (2002): Pages 211-215.
[2] Huang, Zhicheng et al. “Enhanced thermoelectric performance of p-type Mg2Sn single crystals via multi-scale defect engineering†.” Journal of Materials Chemistry A 6 (2022): 2652–2660.